Protein redox modification as a cellular defense mechanism against tissue ischemic injury
- PMID: 24883175
- PMCID: PMC4026984
- DOI: 10.1155/2014/343154
Protein redox modification as a cellular defense mechanism against tissue ischemic injury
Abstract
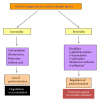
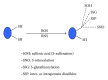
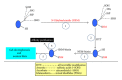
Protein oxidative or redox modifications induced by reactive oxygen species (ROS) or reactive nitrogen species (RNS) not only can impair protein function, but also can regulate and expand protein function under a variety of stressful conditions. Protein oxidative modifications can generally be classified into two categories: irreversible oxidation and reversible oxidation. While irreversible oxidation usually leads to protein aggregation and degradation, reversible oxidation that usually occurs on protein cysteine residues can often serve as an "on and off" switch that regulates protein function and redox signaling pathways upon stress challenges. In the context of ischemic tolerance, including preconditioning and postconditioning, increasing evidence has indicated that reversible cysteine redox modifications such as S-sulfonation, S-nitrosylation, S-glutathionylation, and disulfide bond formation can serve as a cellular defense mechanism against tissue ischemic injury. In this review, I highlight evidence of cysteine redox modifications as protective measures in ischemic injury, demonstrating that protein redox modifications can serve as a therapeutic target for attenuating tissue ischemic injury. Prospectively, more oxidatively modified proteins will need to be identified that can play protective roles in tissue ischemic injury, in particular, when the oxidative modifications of such identified proteins can be enhanced by pharmacological agents or drugs that are available or to be developed.
Figures
Similar articles
-
Redox proteomics: from bench to bedside.Adv Exp Med Biol. 2014;806:301-17. doi: 10.1007/978-3-319-06068-2_13. Adv Exp Med Biol. 2014. PMID: 24952188 Review.
-
Pathological Impact of Redox Post-Translational Modifications.Antioxid Redox Signal. 2024 Jul;41(1-3):152-180. doi: 10.1089/ars.2023.0252. Epub 2024 Apr 8. Antioxid Redox Signal. 2024. PMID: 38504589 Review.
-
A direct way of redox sensing.RNA Biol. 2011 Jan-Feb;8(1):18-23. doi: 10.4161/rna.8.1.13555. Epub 2011 Jan 1. RNA Biol. 2011. PMID: 21220941
-
ROS and RNS signalling: adaptive redox switches through oxidative/nitrosative protein modifications.Free Radic Res. 2018 May;52(5):507-543. doi: 10.1080/10715762.2018.1457217. Epub 2018 Apr 19. Free Radic Res. 2018. PMID: 29589770 Review.
-
Introduction to the thematic minireview series on redox-active protein modifications and signaling.J Biol Chem. 2013 Sep 13;288(37):26463. doi: 10.1074/jbc.R113.502583. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861402 Free PMC article.
Cited by
-
How to Improve the Antioxidant Defense in Asphyxiated Newborns-Lessons from Animal Models.Antioxidants (Basel). 2020 Sep 21;9(9):898. doi: 10.3390/antiox9090898. Antioxidants (Basel). 2020. PMID: 32967335 Free PMC article. Review.
-
"Oxygen Sensing" by Na,K-ATPase: These Miraculous Thiols.Front Physiol. 2016 Aug 2;7:314. doi: 10.3389/fphys.2016.00314. eCollection 2016. Front Physiol. 2016. PMID: 27531981 Free PMC article. Review.
-
Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans.Oxid Med Cell Longev. 2017;2017:6501046. doi: 10.1155/2017/6501046. Epub 2017 Jun 18. Oxid Med Cell Longev. 2017. PMID: 28698768 Free PMC article. Review.
-
Differential Post-Translational Modifications of Proteins in Bladder Ischemia.Biomedicines. 2023 Dec 28;12(1):81. doi: 10.3390/biomedicines12010081. Biomedicines. 2023. PMID: 38255188 Free PMC article.
-
Characteristic of the Oxidative Stress in Blood of Patients in Dependence of Community-Acquired Pneumonia Severity.Open Access Maced J Med Sci. 2016 Mar 15;4(1):122-7. doi: 10.3889/oamjms.2016.040. Epub 2016 Mar 11. Open Access Maced J Med Sci. 2016. PMID: 27275344 Free PMC article.
References
-
- Groeger G, Quiney C, Cotter TG. Hydrogen peroxide as a cell-survival signaling molecule. Antioxidants and Redox Signaling. 2009;11(11):2655–2671. - PubMed
-
- Groeger G, Doonan F, Cotter TG, Donovan M. Reactive oxygen species regulate prosurvival ERK1/2 signaling and bFGF expression in gliosis within the retina. Investigative Ophthalmology & Visual Science. 2012;53:6645–6654. - PubMed
-
- Bevilacqua E, Gomes SZ, Lorenzon AR, Hoshida MS, Amarante-Paffaro AM. NADPH oxidase as an important source of reactive oxygen species at the mouse maternal-fetal interface: putative biological roles. Reproductive BioMedicine Online. 2012;25:31–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

