Functional insight into development of positive allosteric modulators of AMPA receptors
- PMID: 24878241
- PMCID: PMC4107126
- DOI: 10.1016/j.neuropharm.2014.05.022
Functional insight into development of positive allosteric modulators of AMPA receptors
Abstract
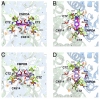
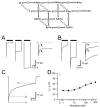
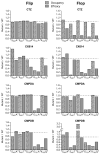
Positive allosteric modulators of α-amino-3-hydroxy-5-methyl-isoxazole-propionic acid (AMPA) ionotropic glutamate receptors facilitate synaptic plasticity and contribute essentially to learning and memory, properties which make AMPA receptors targets for drug discovery and development. One region at which several different classes of positive allosteric modulators bind lies at the dimer interface between the ligand-binding core of the second, membrane-proximal, extracellular domain of AMPA receptors. This solvent-accessible binding pocket has been the target of drug discovery efforts, leading to the recent delineation of five "subsites" which differentially allow access to modulator moieties, and for which distinct modulator affinities and apparent efficacies are attributed. Here we use the voltage-clamp technique in conjunction with rapid drug application to study the effects of mutants lining subsites "A" and "B" of the allosteric modulator pocket to assess affinity and efficacy of allosteric modulation by cyclothiazide, CX614, CMPDA and CMPDB. A novel analysis of the decay of current produced by the onset of desensitization has allowed us to estimate both affinity and efficacy from single concentrations of modulator. Such an approach may be useful for effective high throughput screening of new target compounds.
Keywords: AMPAkine; Allosteric modulation; Deactivation; Desensitization; Electrophysiology; Ion channel gating; Mutagenesis; Neuropharmacology.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Structural and functional analysis of two new positive allosteric modulators of GluA2 desensitization and deactivation.Mol Pharmacol. 2011 Aug;80(2):267-80. doi: 10.1124/mol.110.070243. Epub 2011 May 4. Mol Pharmacol. 2011. PMID: 21543522 Free PMC article.
-
A charge-inverting mutation in the "linker" region of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors alters agonist binding and gating kinetics independently of allosteric modulators.J Biol Chem. 2014 Apr 11;289(15):10702-10714. doi: 10.1074/jbc.M113.526921. Epub 2014 Feb 18. J Biol Chem. 2014. PMID: 24550387 Free PMC article.
-
Stargazin differentially modulates ampakine gating kinetics and pharmacology.Biochem Pharmacol. 2018 Feb;148:308-314. doi: 10.1016/j.bcp.2018.01.019. Epub 2018 Jan 9. Biochem Pharmacol. 2018. PMID: 29330065
-
Allosteric regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate receptors by thiocyanate and cyclothiazide at a common modulatory site distinct from that of 2,3-benzodiazepines.Neuroscience. 1998 Dec;87(3):615-29. doi: 10.1016/s0306-4522(98)00109-2. Neuroscience. 1998. PMID: 9758228
-
AMPA receptor neurotransmission and therapeutic applications: A comprehensive review of their multifaceted modulation.Eur J Med Chem. 2024 Feb 15;266:116151. doi: 10.1016/j.ejmech.2024.116151. Epub 2024 Jan 14. Eur J Med Chem. 2024. PMID: 38237342 Review.
Cited by
-
The influence of levetiracetam in cognitive performance in healthy individuals: neuropsychological, behavioral and electrophysiological approach.Clin Psychopharmacol Neurosci. 2015 Apr 30;13(1):83-93. doi: 10.9758/cpn.2015.13.1.83. Clin Psychopharmacol Neurosci. 2015. PMID: 25912541 Free PMC article.
-
AMPA receptor potentiators: from drug design to cognitive enhancement.Curr Opin Pharmacol. 2015 Feb;20:46-53. doi: 10.1016/j.coph.2014.11.002. Epub 2014 Nov 27. Curr Opin Pharmacol. 2015. PMID: 25462292 Free PMC article. Review.
-
Crystal Structures of Potent Dimeric Positive Allosteric Modulators at the Ligand-Binding Domain of the GluA2 Receptor.ACS Med Chem Lett. 2018 Nov 4;10(3):243-247. doi: 10.1021/acsmedchemlett.8b00369. eCollection 2019 Mar 14. ACS Med Chem Lett. 2018. PMID: 30891120 Free PMC article.
-
The Impact and Mechanism of a Novel Allosteric AMPA Receptor Modulator LCX001 on Protection Against Respiratory Depression in Rodents.Front Pharmacol. 2019 Feb 19;10:105. doi: 10.3389/fphar.2019.00105. eCollection 2019. Front Pharmacol. 2019. PMID: 30837875 Free PMC article.
-
Rational Design of a Novel AMPA Receptor Modulator through a Hybridization Approach.ACS Med Chem Lett. 2015 Feb 11;6(4):392-6. doi: 10.1021/ml5004553. eCollection 2015 Apr 9. ACS Med Chem Lett. 2015. PMID: 25893038 Free PMC article.
References
-
- Arai A, Kessler M, Rogers G, Lynch G. Effects of the potent ampakine CX614 on hippocampal and recombinant AMPA receptors: interactions with cyclothiazide and GYKI 52466. Molecular Pharmacology. 2000;58:802–813. - PubMed
-
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

