Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice
- PMID: 24876275
- PMCID: PMC4066522
- DOI: 10.1073/pnas.1408233111
Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice
Abstract
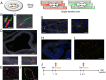
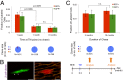
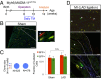
The mammalian heart has long been considered a postmitotic organ, implying that the total number of cardiomyocytes is set at birth. Analysis of cell division in the mammalian heart is complicated by cardiomyocyte binucleation shortly after birth, which makes it challenging to interpret traditional assays of cell turnover [Laflamme MA, Murray CE (2011) Nature 473(7347):326-335; Bergmann O, et al. (2009) Science 324(5923):98-102]. An elegant multi-isotope imaging-mass spectrometry technique recently calculated the low, discrete rate of cardiomyocyte generation in mice [Senyo SE, et al. (2013) Nature 493(7432):433-436], yet our cellular-level understanding of postnatal cardiomyogenesis remains limited. Herein, we provide a new line of evidence for the differentiated α-myosin heavy chain-expressing cardiomyocyte as the cell of origin of postnatal cardiomyogenesis using the "mosaic analysis with double markers" mouse model. We show limited, life-long, symmetric division of cardiomyocytes as a rare event that is evident in utero but significantly diminishes after the first month of life in mice; daughter cardiomyocytes divide very seldom, which this study is the first to demonstrate, to our knowledge. Furthermore, ligation of the left anterior descending coronary artery, which causes a myocardial infarction in the mosaic analysis with double-marker mice, did not increase the rate of cardiomyocyte division above the basal level for up to 4 wk after the injury. The clonal analysis described here provides direct evidence of postnatal mammalian cardiomyogenesis.
Keywords: aging; cardiovascular progenitors; heart development; regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Redox Regulation of Heart Regeneration: An Evolutionary Tradeoff.Front Cell Dev Biol. 2016 Dec 15;4:137. doi: 10.3389/fcell.2016.00137. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 28018900 Free PMC article.
-
Cardiomyocyte cell cycle dynamics and proliferation revealed through cardiac-specific transgenesis of fluorescent ubiquitinated cell cycle indicator (FUCCI).J Mol Cell Cardiol. 2019 Feb;127:154-164. doi: 10.1016/j.yjmcc.2018.12.007. Epub 2018 Dec 18. J Mol Cell Cardiol. 2019. PMID: 30571978 Free PMC article.
-
Midbody Positioning and Distance Between Daughter Nuclei Enable Unequivocal Identification of Cardiomyocyte Cell Division in Mice.Circ Res. 2018 Oct 12;123(9):1039-1052. doi: 10.1161/CIRCRESAHA.118.312792. Circ Res. 2018. PMID: 30355161
-
Reviewing the Limitations of Adult Mammalian Cardiac Regeneration: Noncoding RNAs as Regulators of Cardiomyogenesis.Biomolecules. 2020 Feb 10;10(2):262. doi: 10.3390/biom10020262. Biomolecules. 2020. PMID: 32050588 Free PMC article. Review.
-
Role of Mononuclear Cardiomyocytes in Cardiac Turnover and Regeneration.Curr Cardiol Rep. 2020 May 19;22(6):39. doi: 10.1007/s11886-020-01289-y. Curr Cardiol Rep. 2020. PMID: 32430578 Free PMC article. Review.
Cited by
-
Heart Regeneration by Endogenous Stem Cells and Cardiomyocyte Proliferation: Controversy, Fallacy, and Progress.Circulation. 2020 Jul 21;142(3):275-291. doi: 10.1161/CIRCULATIONAHA.119.045566. Epub 2020 Jul 20. Circulation. 2020. PMID: 32687441 Free PMC article.
-
Cardiac Regeneration and Stem Cells.Physiol Rev. 2015 Oct;95(4):1189-204. doi: 10.1152/physrev.00021.2014. Physiol Rev. 2015. PMID: 26269526 Free PMC article. Review.
-
Parallels between vertebrate cardiac and cutaneous wound healing and regeneration.NPJ Regen Med. 2018 Nov 7;3:21. doi: 10.1038/s41536-018-0059-y. eCollection 2018. NPJ Regen Med. 2018. PMID: 30416753 Free PMC article. Review.
-
Length and secondary structure of the 5' non-coding regions of mouse p53 mRNA transcripts - mouse as a model organism for p53 gene expression studies.RNA Biol. 2019 Jan;16(1):25-41. doi: 10.1080/15476286.2018.1556084. Epub 2018 Dec 20. RNA Biol. 2019. PMID: 30518296 Free PMC article.
-
Molecular mechanisms of heart regeneration.Semin Cell Dev Biol. 2020 Apr;100:20-28. doi: 10.1016/j.semcdb.2019.09.003. Epub 2019 Oct 4. Semin Cell Dev Biol. 2020. PMID: 31587963 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

