Low-dose 5-aza-2'-deoxycytidine pretreatment inhibits experimental autoimmune encephalomyelitis by induction of regulatory T cells
- PMID: 24869907
- PMCID: PMC4107100
- DOI: 10.2119/molmed.2013.00159
Low-dose 5-aza-2'-deoxycytidine pretreatment inhibits experimental autoimmune encephalomyelitis by induction of regulatory T cells
Abstract
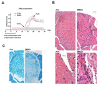
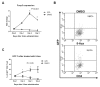
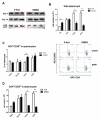
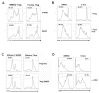
Forkhead box P3 (Foxp3) is the major transcription factor controlling the development and function of regulatory T (Treg) cells. Previous studies have indicated epigenetic regulation of Foxp3 expression. Here, we investigated whether the deoxyribonucleic acid (DNA) methyltransferase inhibitor 5-aza-2'-deoxycytidine (5-Aza) applied peripherally could modulate central nervous system (CNS) inflammation, by using a mouse experimental autoimmune encephalomyelitis (EAE) model. We found that disease activity was inhibited in a myelin oligodendrocyte glycoprotein (MOG) peptide-induced EAE mouse briefly pretreated with low-dose (0.15 mg/kg) 5-Aza, ameliorating significant CNS inflammatory responses, as indicated by greatly decreased proinflammatory cytokines. On the contrary, control EAE mice expressed high levels of IFN-γ and interleukin (IL)-17. In addition, 5-Aza treatment in vitro increased GFP expression in CD4(+)GFP(-) T cells isolated from GFP knock-in Foxp3 transgenic mice. Importantly, 5-Aza treatment increased Treg cell numbers, in EAE mice, at both disease onset and peak. However, Treg inhibition assays showed 5-Aza treatment did not enhance per-cell Treg inhibitory function, but did maintain a lower activation threshold for effector cells in EAE mice. In conclusion, 5-Aza treatment prevented EAE development and suppressed CNS inflammation, by increasing the number of Treg cells and inhibiting effector cells in the periphery.
Figures

Similar articles
-
Mitigation of experimental allergic encephalomyelitis by TGF-beta induced Foxp3+ regulatory T lymphocytes through the induction of anergy and infectious tolerance.J Immunol. 2008 Mar 1;180(5):2830-8. doi: 10.4049/jimmunol.180.5.2830. J Immunol. 2008. PMID: 18292504
-
IFN-β Facilitates Neuroantigen-Dependent Induction of CD25+ FOXP3+ Regulatory T Cells That Suppress Experimental Autoimmune Encephalomyelitis.J Immunol. 2016 Oct 15;197(8):2992-3007. doi: 10.4049/jimmunol.1500411. Epub 2016 Sep 12. J Immunol. 2016. PMID: 27619998 Free PMC article.
-
Treatment with MOG-DNA vaccines induces CD4+CD25+FoxP3+ regulatory T cells and up-regulates genes with neuroprotective functions in experimental autoimmune encephalomyelitis.J Neuroinflammation. 2012 Jun 22;9:139. doi: 10.1186/1742-2094-9-139. J Neuroinflammation. 2012. PMID: 22727044 Free PMC article.
-
A GMCSF-Neuroantigen Tolerogenic Vaccine Elicits Systemic Lymphocytosis of CD4+ CD25high FOXP3+ Regulatory T Cells in Myelin-Specific TCR Transgenic Mice Contingent Upon Low-Efficiency T Cell Antigen Receptor Recognition.Front Immunol. 2019 Jan 10;9:3119. doi: 10.3389/fimmu.2018.03119. eCollection 2018. Front Immunol. 2019. PMID: 30687323 Free PMC article.
-
Involvement of regulatory T cells in the experimental autoimmune encephalomyelitis-preventive effect of dendritic cells expressing myelin oligodendrocyte glycoprotein plus TRAIL.J Immunol. 2007 Jan 15;178(2):918-25. doi: 10.4049/jimmunol.178.2.918. J Immunol. 2007. PMID: 17202353
Cited by
-
Epigenetic regulation of B cells and its role in autoimmune pathogenesis.Cell Mol Immunol. 2022 Nov;19(11):1215-1234. doi: 10.1038/s41423-022-00933-7. Epub 2022 Oct 12. Cell Mol Immunol. 2022. PMID: 36220996 Free PMC article. Review.
-
Non-Coding RNA in Systemic Sclerosis: A Valuable Tool for Translational and Personalized Medicine.Genes (Basel). 2021 Aug 24;12(9):1296. doi: 10.3390/genes12091296. Genes (Basel). 2021. PMID: 34573278 Free PMC article. Review.
-
Decitabine inhibits T cell proliferation via a novel TET2-dependent mechanism and exerts potent protective effect in mouse auto- and allo-immunity models.Oncotarget. 2017 May 22;8(34):56802-56815. doi: 10.18632/oncotarget.18063. eCollection 2017 Aug 22. Oncotarget. 2017. PMID: 28915632 Free PMC article.
-
Augmenting chemotherapy with low-dose decitabine through an immune-independent mechanism.JCI Insight. 2022 Nov 22;7(22):e159419. doi: 10.1172/jci.insight.159419. JCI Insight. 2022. PMID: 36227698 Free PMC article.
-
m6A RNA Methylation in Systemic Autoimmune Diseases-A New Target for Epigenetic-Based Therapy?Pharmaceuticals (Basel). 2021 Mar 5;14(3):218. doi: 10.3390/ph14030218. Pharmaceuticals (Basel). 2021. PMID: 33807762 Free PMC article. Review.
References
-
- Pachner AR. Experimental models of multiple sclerosis. Curr Opin Neurol. 2011;24:291–99. - PubMed
-
- Kroenke MA, Segal BM. Th17 and Th1 responses directed against the immunizing epi-tope, as opposed to secondary epitopes, dominate the autoimmune repertoire during relapses of experimental autoimmune encephalomyelitis. J Neurosci Res. 2007;85:1685–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

