Coprescription of Chinese herbal medicine and Western medication among female patients with breast cancer in Taiwan: analysis of national insurance claims
- PMID: 24855343
- PMCID: PMC4019611
- DOI: 10.2147/PPA.S61280
Coprescription of Chinese herbal medicine and Western medication among female patients with breast cancer in Taiwan: analysis of national insurance claims
Abstract
Background: Many female breast cancer (FBC) patients take Chinese herbal medicine (CHM) and Western medication (WM) concurrently in Taiwan. Despite the possibility of interactions between the CHM and WM mentioned in previous studies, the pattern of these coprescriptions in FBC patients remains unclear. Hence, the aim of the present study is to investigate the utilization of coprescriptions of CHM and WM among the FBC patients in Taiwan.
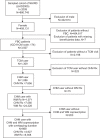
Methods: The study was a cross-sectional survey using the sampled cohort in 2009 obtained from the National Health Insurance Research Database in Taiwan. There were 3,507 FBC patients identified from the registry for catastrophic illness patients. Ambulatory visit records, corresponding prescriptions, and the data of beneficiaries belonging to the FBC patients were further extracted. A total of 1,086 FBC patients used CHM at least once. CHM and WM prescribed within any overlapping duration were defined as coprescriptions.
Results: There were 868 (80.0%) patients simultaneously receiving CHM and WM. A total of 4,927 CHM prescriptions and 6,358 WM prescriptions were prescribed concurrently. Among these coprescriptions, the most frequently used CHM was jia-wei-xiao-yao-san (21.2%), and the most frequently coprescribed WM was acetaminophen (38.9%), followed by tamoxifen (25.5%). There were 346 patients using systemic adjuvant therapy and CHM concurrently. The most commonly coprescribed CHM with chemotherapy, endocrine therapy, and trastuzumab was xiang-sha-liu-jun-zi-tang, jia-wei-xiao-yao-san, and zhi-gan-cao-tang, respectively.
Conclusion: The combined use of CHM with WM is prevalent. The main purpose of combining CHM with systemic cancer treatment is to alleviate the treatment-related adverse effects. However, the combination may result in the potential risk of drug-herb interactions. Further clinical studies are needed to evaluate the efficacy and safety of the CHM and WM coprescriptions for FBC patients.
Keywords: complementary and alternative medicine; drug utilization patterns; pharmacoepidemiology.
Figures
Similar articles
-
Coprescription of Chinese Herbal Medicine and Western Medications among Prostate Cancer Patients: A Population-Based Study in Taiwan.Evid Based Complement Alternat Med. 2012;2012:147015. doi: 10.1155/2012/147015. Epub 2011 Jul 18. Evid Based Complement Alternat Med. 2012. PMID: 21792368 Free PMC article.
-
Prescription patterns and factors influencing the use of Chinese herbal medicine among pregnant women in Taiwan: a population-based retrospective study.BMC Complement Med Ther. 2020 Jul 30;20(1):240. doi: 10.1186/s12906-020-03032-0. BMC Complement Med Ther. 2020. PMID: 32731888 Free PMC article.
-
Complementary Chinese herbal medicine therapy improves survival of patients with gastric cancer in Taiwan: A nationwide retrospective matched-cohort study.J Ethnopharmacol. 2017 Mar 6;199:168-174. doi: 10.1016/j.jep.2017.02.004. Epub 2017 Feb 3. J Ethnopharmacol. 2017. PMID: 28163114
-
Concurrent administration of anticancer chemotherapy drug and herbal medicine on the perspective of pharmacokinetics.J Food Drug Anal. 2018 Apr;26(2S):S88-S95. doi: 10.1016/j.jfda.2018.01.003. Epub 2018 Feb 3. J Food Drug Anal. 2018. PMID: 29703390 Free PMC article. Review.
-
[Systematic review and meta-analysis of randomized controlled trials of Chinese herbal medicine in the treatment of Sjogren's syndrome].Zhong Xi Yi Jie He Xue Bao. 2011 Mar;9(3):257-74. doi: 10.3736/jcim20110306. Zhong Xi Yi Jie He Xue Bao. 2011. PMID: 21419078 Review. Chinese.
Cited by
-
Chemopreventive effects of Xiang Sha Liu Jun Zi Tang on paclitaxel-induced leucopenia and neuropathy in animals.Front Pharmacol. 2023 Mar 2;14:1106030. doi: 10.3389/fphar.2023.1106030. eCollection 2023. Front Pharmacol. 2023. PMID: 36969850 Free PMC article.
-
Effects of Chinese herbal medicines on the occurrence of diabetic retinopathy in type 2 diabetes patients and protection of ARPE-19 retina cells by inhibiting oxidative stress.Oncotarget. 2017 Jun 29;8(38):63528-63550. doi: 10.18632/oncotarget.18846. eCollection 2017 Sep 8. Oncotarget. 2017. PMID: 28969009 Free PMC article.
-
Traditional Chinese Medicine Treatment Associated with Female Infertility in Taiwan: A Population-Based Case-Control Study.Evid Based Complement Alternat Med. 2020 Dec 8;2020:3951741. doi: 10.1155/2020/3951741. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33381200 Free PMC article.
-
Chinese Herbal Medicine as an Adjunctive Therapy Ameliorated the Incidence of Chronic Hepatitis in Patients with Breast Cancer: A Nationwide Population-Based Cohort Study.Evid Based Complement Alternat Med. 2017;2017:1052976. doi: 10.1155/2017/1052976. Epub 2017 Oct 30. Evid Based Complement Alternat Med. 2017. PMID: 29234362 Free PMC article.
-
Complementary and Alternative Medicine Use in Breast Cancer Patients at a Medical Center in Taiwan: A Cross-Sectional Study.Integr Cancer Ther. 2020 Jan-Dec;19:1534735420983910. doi: 10.1177/1534735420983910. Integr Cancer Ther. 2020. PMID: 33372560 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Bureau of Health Promotion, Department of Health Cancer registry annual report, 2010, Taiwan. 2013. [Accessed April 29, 2014]. Available from: http://www.hpa.gov.tw/BHPNet/Portal/File/StatisticsFile/2013050610370652....
-
- Berry DA, Cronin KA, Plevritis SK, et al. Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. - PubMed
-
- Howell A, Cuzick J, Baum M, et al. ATAC Trialists’ Group Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–62. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

