Changes in input strength and number are driven by distinct mechanisms at the retinogeniculate synapse
- PMID: 24848465
- PMCID: PMC4122736
- DOI: 10.1152/jn.00175.2014
Changes in input strength and number are driven by distinct mechanisms at the retinogeniculate synapse
Abstract
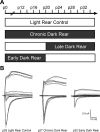
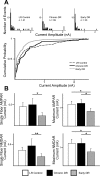
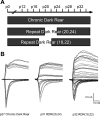
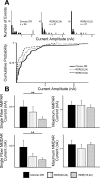
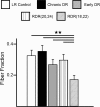
Recent studies have demonstrated that vision influences the functional remodeling of the mouse retinogeniculate synapse, the connection between retinal ganglion cells and thalamic relay neurons in the dorsal lateral geniculate nucleus (LGN). Initially, each relay neuron receives a large number of weak retinal inputs. Over a 2- to 3-wk developmental window, the majority of these inputs are eliminated, and the remaining inputs are strengthened. This period of refinement is followed by a critical period when visual experience changes the strength and connectivity of the retinogeniculate synapse. Visual deprivation of mice by dark rearing from postnatal day (P)20 results in a dramatic weakening of synaptic strength and recruitment of additional inputs. In the present study we asked whether experience-dependent plasticity at the retinogeniculate synapse represents a homeostatic response to changing visual environment. We found that visual experience starting at P20 following visual deprivation from birth results in weakening of existing retinal inputs onto relay neurons without significant changes in input number, consistent with homeostatic synaptic scaling of retinal inputs. On the other hand, the recruitment of new inputs to the retinogeniculate synapse requires previous visual experience prior to the critical period. Taken together, these findings suggest that diverse forms of homeostatic plasticity drive experience-dependent remodeling at the retinogeniculate synapse.
Keywords: critical period; synapse development; synaptic plasticity; thalamus; vision.
Copyright © 2014 the American Physiological Society.
Figures

Similar articles
-
An evolving view of retinogeniculate transmission.Vis Neurosci. 2017 Jan;34:E013. doi: 10.1017/S0952523817000104. Vis Neurosci. 2017. PMID: 28965513 Free PMC article. Review.
-
Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse.J Neurosci. 2008 Apr 30;28(18):4807-17. doi: 10.1523/JNEUROSCI.4667-07.2008. J Neurosci. 2008. PMID: 18448657 Free PMC article.
-
Functional Convergence at the Retinogeniculate Synapse.Neuron. 2017 Oct 11;96(2):330-338.e5. doi: 10.1016/j.neuron.2017.09.037. Neuron. 2017. PMID: 29024658 Free PMC article.
-
Developmental remodeling of the retinogeniculate synapse.Neuron. 2000 Dec;28(3):955-66. doi: 10.1016/s0896-6273(00)00166-5. Neuron. 2000. PMID: 11163279
-
Organization, Function, and Development of the Mouse Retinogeniculate Synapse.Annu Rev Vis Sci. 2020 Sep 15;6:261-285. doi: 10.1146/annurev-vision-121219-081753. Annu Rev Vis Sci. 2020. PMID: 32936733 Review.
Cited by
-
Early-Stage Ocular Hypertension Alters Retinal Ganglion Cell Synaptic Transmission in the Visual Thalamus.Front Cell Neurosci. 2019 Sep 19;13:426. doi: 10.3389/fncel.2019.00426. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31607867 Free PMC article.
-
Homeostatic plasticity in neural development.Neural Dev. 2018 Jun 1;13(1):9. doi: 10.1186/s13064-018-0105-x. Neural Dev. 2018. PMID: 29855353 Free PMC article. Review.
-
An evolving view of retinogeniculate transmission.Vis Neurosci. 2017 Jan;34:E013. doi: 10.1017/S0952523817000104. Vis Neurosci. 2017. PMID: 28965513 Free PMC article. Review.
-
Cortical Feedback Regulates Feedforward Retinogeniculate Refinement.Neuron. 2016 Sep 7;91(5):1021-1033. doi: 10.1016/j.neuron.2016.07.040. Epub 2016 Aug 18. Neuron. 2016. PMID: 27545712 Free PMC article.
-
Sensory Activity-Dependent and Sensory Activity-Independent Properties of the Developing Rodent Trigeminal Principal Nucleus.Dev Neurosci. 2016;38(3):163-170. doi: 10.1159/000446395. Epub 2016 Jun 9. Dev Neurosci. 2016. PMID: 27287019 Free PMC article. Review.
References
-
- Carrasco MM, Razak KA, Pallas SL. Visual experience is necessary for maintenance but not development of receptive fields in superior colliculus. J Neurophysiol 94: 1962–1970, 2005 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

