Thiazolidinedione-independent activation of peroxisome proliferator-activated receptor γ is a potential target for diabetic macrovascular complications
- PMID: 24843540
- PMCID: PMC4014927
- DOI: 10.1111/j.2040-1124.2011.00182.x
Thiazolidinedione-independent activation of peroxisome proliferator-activated receptor γ is a potential target for diabetic macrovascular complications
Abstract
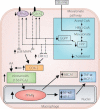
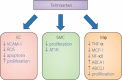
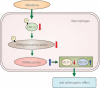
Macrovascular complications are responsible for the high morbidity and mortality in patients with diabetes. Peroxisome proliferator-activated receptor γ (PPARγ) plays a central role in the process of adipocyte differentiation and insulin sensitization, and also possesses anti-atherogenic effects. Recently, some statins, angiotensin II type 1 receptor blockers and calcium channel blockers have been reported to activate PPARγ. However, the impact of PPARγ activation on diabetic macrovascular complications is not fully understood. It has been reported that the activation of PPARγ by thiazolidinediones induces anti-atherogenic effects in vascular cells, including monocytes/macrophages, endothelial cells and smooth muscle cells, in atherosclerotic animal models and in clinical studies. We have reported that hydroxymethylglutaryl coenzyme A reductase inhibitors (statins), which are used for treatment of hypercholesterolemia, activate PPARγ and mediate anti-atherogenic effects through PPARγ activation in macrophages. Also, telmisartan, an angiotensin type I receptor blocker, has been reported to have anti-atherogenic effects through PPARγ activation. Furthermore, we have reported that nifedipine, a dihydropyridine calcium channel blocker, can activate PPARγ, thereby mediating anti-atherogenic effects in macrophages. Therefore, statin therapy and part of anti-hypertensive therapy might produce beneficial effects through PPARγ activation in hypercholesterolemic and/or hypertensive patients with diabetes, and PPARγ might be a therapeutic target for diabetic macrovascular complications. In the present review, we focus on the anti-atherogenic effects of PPARγ and suggest potential therapeutic approaches to prevent diabetic macrovascular complications. (J Diabetes Invest, doi: 10.1111/j.2040-1124.2011.00182.x, 2012).
Keywords: Diabetes; Macroangiopathy; Peroxisome proliferator‐activated receptor γ.
Figures
Similar articles
-
Statins meditate anti-atherosclerotic action in smooth muscle cells by peroxisome proliferator-activated receptor-γ activation.Biochem Biophys Res Commun. 2015 Jan 30;457(1):23-30. doi: 10.1016/j.bbrc.2014.12.063. Epub 2014 Dec 19. Biochem Biophys Res Commun. 2015. PMID: 25529449
-
Telmisartan protects against diabetic vascular complications in a mouse model of obesity and type 2 diabetes, partially through peroxisome proliferator activated receptor-γ-dependent activity.Biochem Biophys Res Commun. 2011 Jul 8;410(3):508-13. doi: 10.1016/j.bbrc.2011.06.012. Epub 2011 Jun 7. Biochem Biophys Res Commun. 2011. PMID: 21679694
-
Nifedipine induces peroxisome proliferator-activated receptor-gamma activation in macrophages and suppresses the progression of atherosclerosis in apolipoprotein E-deficient mice.Arterioscler Thromb Vasc Biol. 2010 Aug;30(8):1598-605. doi: 10.1161/ATVBAHA.109.202309. Epub 2010 May 27. Arterioscler Thromb Vasc Biol. 2010. PMID: 20508203
-
The Role of Peroxisome Proliferator-Activated Receptor Gamma and Atherosclerosis: Post-translational Modification and Selective Modulators.Front Physiol. 2022 Mar 2;13:826811. doi: 10.3389/fphys.2022.826811. eCollection 2022. Front Physiol. 2022. PMID: 35309069 Free PMC article. Review.
-
Inflammation in diabetes mellitus: role of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma agonists.Am J Cardiol. 2007 Feb 19;99(4A):27B-40B. doi: 10.1016/j.amjcard.2006.11.004. Epub 2006 Dec 22. Am J Cardiol. 2007. PMID: 17307056 Review.
References
-
- Abbott RD, Donahue RP, Kannel WB, et al. The impact of diabetes on survival following myocardial infarction in men vs women. The Framingham Study. JAMA 1988; 260: 3456–3460 - PubMed
-
- Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the US population, 1971–1993. Diabetes Care 1998; 21: 1138–1145 - PubMed
-
- Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and non‐diabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229–234 - PubMed
-
- Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham Study. JAMA 1979; 241: 2035–2038 - PubMed
-
- Pan WH, Cedres LB, Liu K, et al. Relationship of clinical diabetes and asymptomatic hyperglycemia to risk of coronary heart disease mortality in men and women. Am J Epidemiol 1986; 123: 504–516 - PubMed
Publication types
LinkOut - more resources
Full Text Sources

