A phenotypic high throughput screening assay for the identification of pharmacoperones for the gonadotropin releasing hormone receptor
- PMID: 24831790
- PMCID: PMC4025569
- DOI: 10.1089/adt.2014.576
A phenotypic high throughput screening assay for the identification of pharmacoperones for the gonadotropin releasing hormone receptor
Abstract
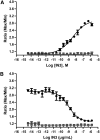
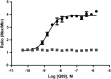
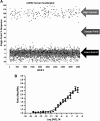
We describe a phenotypic high throughput screening (HTS) calcium flux assay designed to identify pharmacoperones for the gonadotropin releasing hormone receptor (GnRHR). Pharmacoperones are target-specific, small molecules that diffuse into cells, rescue misfolded protein mutants, and restore them to function. Rescue is based on correcting the trafficking of mutants that would otherwise be retained in the endoplasmic reticulum and unable to function correctly. This approach identifies drugs with a significant degree of novelty, relying on cellular mechanisms that are not currently exploited. Development of such assays is important, since the extensive use of agonist/antagonist screens alone means that useful chemical structures may be present in existing libraries but have not been previously identified using existing methods. Our assay utilizes cell lines stably expressing a GnRHR mutant under the control of a tetracycline (OFF) transactivator. This allows us to quantitate the level of functional and properly trafficked G protein coupled receptors present in each test well. Furthermore, since we are able to turn receptor expression on and off, we can rapidly eliminate the majority of false positives from our screening results. Our data show that this approach is likely to be successful in identifying hits from large chemical libraries.
Figures
Similar articles
-
Rescue of mutant gonadotropin-releasing hormone receptor function independent of cognate receptor activity.Sci Rep. 2020 Jun 29;10(1):10579. doi: 10.1038/s41598-020-67473-w. Sci Rep. 2020. PMID: 32601341 Free PMC article.
-
Therapeutic rescue of misfolded mutants: validation of primary high throughput screens for identification of pharmacoperone drugs.PLoS One. 2011;6(7):e22784. doi: 10.1371/journal.pone.0022784. Epub 2011 Jul 27. PLoS One. 2011. PMID: 21818389 Free PMC article.
-
Therapeutic rescue of misfolded/mistrafficked mutants: automation-friendly high-throughput assays for identification of pharmacoperone drugs of GPCRs.Methods Enzymol. 2013;521:3-16. doi: 10.1016/B978-0-12-391862-8.00001-6. Methods Enzymol. 2013. PMID: 23351731
-
Pharmacological chaperones correct misfolded GPCRs and rescue function: protein trafficking as a therapeutic target.Subcell Biochem. 2012;63:263-89. doi: 10.1007/978-94-007-4765-4_14. Subcell Biochem. 2012. PMID: 23161143 Review.
-
Transitioning pharmacoperones to therapeutic use: in vivo proof-of-principle and design of high throughput screens.Pharmacol Res. 2014 May;83:38-51. doi: 10.1016/j.phrs.2013.12.004. Epub 2013 Dec 25. Pharmacol Res. 2014. PMID: 24373832 Free PMC article. Review.
Cited by
-
Recent Advances and Trends in Chemical CPP-Drug Conjugation Techniques.Molecules. 2021 Mar 13;26(6):1591. doi: 10.3390/molecules26061591. Molecules. 2021. PMID: 33805680 Free PMC article. Review.
-
Assay strategies for identification of therapeutic leads that target protein trafficking.Trends Pharmacol Sci. 2015 Aug;36(8):498-505. doi: 10.1016/j.tips.2015.05.004. Epub 2015 Jun 8. Trends Pharmacol Sci. 2015. PMID: 26067100 Free PMC article. Review.
-
Pharmacoperones as Novel Therapeutics for Diverse Protein Conformational Diseases.Physiol Rev. 2018 Apr 1;98(2):697-725. doi: 10.1152/physrev.00029.2016. Physiol Rev. 2018. PMID: 29442594 Free PMC article. Review.
-
Rescue of mutant gonadotropin-releasing hormone receptor function independent of cognate receptor activity.Sci Rep. 2020 Jun 29;10(1):10579. doi: 10.1038/s41598-020-67473-w. Sci Rep. 2020. PMID: 32601341 Free PMC article.
-
Folding and Misfolding of Human Membrane Proteins in Health and Disease: From Single Molecules to Cellular Proteostasis.Chem Rev. 2019 May 8;119(9):5537-5606. doi: 10.1021/acs.chemrev.8b00532. Epub 2019 Jan 4. Chem Rev. 2019. PMID: 30608666 Free PMC article.
References
-
- Conn PM, Janovick JA: Drug development and the cellular quality control system. Trends Pharmacol Sci 2009;30:228–233 - PubMed
-
- Antelli A, Baldazzi L, Balsamo A, et al. : Two novel GnRHR gene mutations in two siblings with hypogonadotropic hypogonadism. Eur J Endocrinol 2006;155:201–205 - PubMed
-
- Beranova M, Oliveira LM, Bedecarrats GY, et al. : Prevalence, phenotypic spectrum, and modes of inheritance of gonadotropin-releasing hormone receptor mutations in idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 2001;86:1580–1588 - PubMed
-
- Bhangoo A, Jacobson-Dickman E: The genetics of idiopathic hypogonadotropic hypogonadism: unraveling the biology of human sexual development. Pediatr Endocrinol Rev 2009;6:395–404 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

