T cell receptor signals to NF-κB are transmitted by a cytosolic p62-Bcl10-Malt1-IKK signalosome
- PMID: 24825920
- PMCID: PMC6450650
- DOI: 10.1126/scisignal.2004882
T cell receptor signals to NF-κB are transmitted by a cytosolic p62-Bcl10-Malt1-IKK signalosome
Abstract
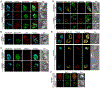
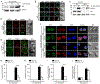
Antigen-mediated stimulation of the T cell receptor (TCR) triggers activation of nuclear factor κB (NF-κB), a key transcriptional regulator of T cell proliferation and effector cell differentiation. TCR signaling to NF-κB requires both the Carma1-Bcl10-Malt1 (CBM) complex and the inhibitor of κB (IκB) kinase (IKK) complex; however, the molecular mechanisms connecting the CBM complex to activation of IKK are incompletely defined. We found that the active IKK complex is a component of a TCR-dependent cytosolic Bcl10-Malt1 signalosome containing the adaptor protein p62, which forms in effector T cells. Phosphorylated IκBα and NF-κB were transiently recruited to this signalosome before NF-κB translocated to the nucleus. Inhibiting the activity of the kinase TAK1 or IKK blocked the phosphorylation of IKK, but not the formation of p62-Bcl10-Malt1 clusters, suggesting that activation of IKK occurs after signalosome assembly. Furthermore, analysis of T cells from p62-deficient mice demonstrated that the p62-dependent clustering of signaling components stimulated activation of NF-κB in effector T cells. Thus, TCR-stimulated activation of NF-κB requires the assembly of cytosolic p62-Bcl10-Malt1-IKK signalosomes, which may ensure highly regulated activation of NF-κB in response to TCR engagement.
Copyright © 2014, American Association for the Advancement of Science.
Figures


Similar articles
-
Protein kinase C-δ negatively regulates T cell receptor-induced NF-κB activation by inhibiting the assembly of CARMA1 signalosome.J Biol Chem. 2012 Jun 8;287(24):20081-7. doi: 10.1074/jbc.M111.335463. Epub 2012 Apr 23. J Biol Chem. 2012. PMID: 22528498 Free PMC article.
-
Selective autophagy of the adaptor protein Bcl10 modulates T cell receptor activation of NF-κB.Immunity. 2012 Jun 29;36(6):947-58. doi: 10.1016/j.immuni.2012.04.008. Epub 2012 May 31. Immunity. 2012. PMID: 22658522 Free PMC article.
-
The Ca2+-dependent phosphatase calcineurin controls the formation of the Carma1-Bcl10-Malt1 complex during T cell receptor-induced NF-kappaB activation.J Biol Chem. 2011 Mar 4;286(9):7522-34. doi: 10.1074/jbc.M110.155895. Epub 2011 Jan 3. J Biol Chem. 2011. PMID: 21199863 Free PMC article.
-
The CARD11-BCL10-MALT1 (CBM) signalosome complex: Stepping into the limelight of human primary immunodeficiency.J Allergy Clin Immunol. 2014 Aug;134(2):276-84. doi: 10.1016/j.jaci.2014.06.015. J Allergy Clin Immunol. 2014. PMID: 25087226 Free PMC article. Review.
-
The CBM signalosome: potential therapeutic target for aggressive lymphoma?Cytokine Growth Factor Rev. 2014 Apr;25(2):175-83. doi: 10.1016/j.cytogfr.2013.12.008. Epub 2013 Dec 24. Cytokine Growth Factor Rev. 2014. PMID: 24411492 Free PMC article. Review.
Cited by
-
Functional characterization of zebrafish (Danio rerio) Bcl10.PLoS One. 2015 Apr 7;10(4):e0122365. doi: 10.1371/journal.pone.0122365. eCollection 2015. PLoS One. 2015. PMID: 25849213 Free PMC article.
-
Altered Lipid Metabolism in Blood Mononuclear Cells of Psoriatic Patients Indicates Differential Changes in Psoriasis Vulgaris and Psoriatic Arthritis.Int J Mol Sci. 2019 Aug 30;20(17):4249. doi: 10.3390/ijms20174249. Int J Mol Sci. 2019. PMID: 31480263 Free PMC article.
-
TCR signaling to NF-κB and mTORC1: Expanding roles of the CARMA1 complex.Mol Immunol. 2015 Dec;68(2 Pt C):546-57. doi: 10.1016/j.molimm.2015.07.024. Epub 2015 Aug 8. Mol Immunol. 2015. PMID: 26260210 Free PMC article. Review.
-
The Many Roles of Ubiquitin in NF-κB Signaling.Biomedicines. 2018 Apr 10;6(2):43. doi: 10.3390/biomedicines6020043. Biomedicines. 2018. PMID: 29642643 Free PMC article. Review.
-
Impaired Control of Epstein-Barr Virus Infection in B-Cell Expansion with NF-κB and T-Cell Anergy Disease.Front Immunol. 2018 Feb 8;9:198. doi: 10.3389/fimmu.2018.00198. eCollection 2018. Front Immunol. 2018. PMID: 29472930 Free PMC article. Review.
References
-
- Gaide O, Favier B, Legler DF, Bonnet D, Brissoni B, Valitutti S, Bron C, Tschopp J, Thome M, CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-kappa B activation. Nat Immunol 3, 836–843 (2002). - PubMed
-
- Bidere N, Snow AL, Sakai K, Zheng L, Lenardo MJ, Caspase-8 regulation by direct interaction with TRAF6 in T cell receptor-induced NF-kappaB activation. Curr Biol 16, 1666–1671 (2006). - PubMed
-
- Sebald A, Mattioli I, Schmitz ML, T cell receptor-induced lipid raft recruitment of the I kappa B kinase complex is necessary and sufficient for NF-kappa B activation occurring in the cytosol. Eur J Immunol 35, 318–325 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

