Biochemical characterization of the castor bean ent-kaurene synthase(-like) family supports quantum chemical view of diterpene cyclization
- PMID: 24810014
- PMCID: PMC4062354
- DOI: 10.1016/j.phytochem.2014.04.005
Biochemical characterization of the castor bean ent-kaurene synthase(-like) family supports quantum chemical view of diterpene cyclization
Abstract
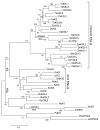
It has become apparent that plants have extensively diversified their arsenal of labdane-related diterpenoids (LRDs), in part via gene duplication and neo-functionalization of the ancestral ent-kaurene synthase (KS) required for gibberellin metabolism. For example, castor bean (Ricinus communis) was previously shown to produce an interesting set of biosynthetically related diterpenes, specifically ent-sandracopimaradiene, ent-beyerene, and ent-trachylobane, in addition to ent-kaurene, using four separate diterpene synthases, albeit these remain unidentified. Notably, despite mechanistic similarity of the underlying reaction to that catalyzed by KSs, ent-beyerene and ent-trachylobane synthases have not yet been identified. Given our interest in LRD biosynthesis, and the recent availability of the castor bean genome sequence, a synthetic biology approach was applied to biochemically characterize the four KS(-like) enzymes [KS(L)s] found in Ricinus communis [i.e., the RcKS(L)s]. In particular, using bacteria engineered to produce the relevant ent-copalyl diphosphate precursor and synthetic genes based on the predicted RcKS(L)s, although this ultimately required correction of a "splicing" error in one of the predicted genes, highlighting the dependence of such a synthetic biology approach on accurate gene sequences. Nevertheless, it is possible to assign each of the four RcKS(L)s to one of the previously observed diterpene synthase activities, providing access to functionally enzymes. Intriguingly, the product distribution of the RcKS(L)s seems to support the distinct diterpene synthase reaction mechanism proposed by quantum chemical calculations, rather than the classically proposed pathway.
Keywords: Diterpenoids; Natural products biosynthesis; Terpene synthases.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
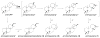
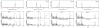


Similar articles
-
Divergent Evolution of the Diterpene Biosynthesis Pathway in Tea Plants (Camellia sinensis) Caused by Single Amino Acid Variation of ent-Kaurene Synthase.J Agric Food Chem. 2020 Sep 16;68(37):9930-9939. doi: 10.1021/acs.jafc.0c03488. Epub 2020 Sep 3. J Agric Food Chem. 2020. PMID: 32841021
-
One amino acid makes the difference: the formation of ent-kaurene and 16α-hydroxy-ent-kaurane by diterpene synthases in poplar.BMC Plant Biol. 2015 Oct 28;15:262. doi: 10.1186/s12870-015-0647-6. BMC Plant Biol. 2015. PMID: 26511849 Free PMC article.
-
Gibberellin-biosynthetic ent-kaurene synthases in higher plants do not require their non-catalytic domains for the catalysis.Biochem J. 2024 Jun 19;481(12):779-791. doi: 10.1042/BCJ20240162. Biochem J. 2024. PMID: 38829839
-
Evolution of Labdane-Related Diterpene Synthases in Cereals.Plant Cell Physiol. 2020 Dec 23;61(11):1850-1859. doi: 10.1093/pcp/pcaa106. Plant Cell Physiol. 2020. PMID: 32810270 Review.
-
To gibberellins and beyond! Surveying the evolution of (di)terpenoid metabolism.Annu Rev Plant Biol. 2014;65:259-86. doi: 10.1146/annurev-arplant-050213-035705. Epub 2014 Jan 22. Annu Rev Plant Biol. 2014. PMID: 24471837 Free PMC article. Review.
Cited by
-
The application of synthetic biology to elucidation of plant mono-, sesqui-, and diterpenoid metabolism.Mol Plant. 2015 Jan;8(1):6-16. doi: 10.1016/j.molp.2014.12.002. Epub 2014 Dec 11. Mol Plant. 2015. PMID: 25578268 Free PMC article. Review.
-
Identification of a novel hedycaryol synthase gene isolated from Camellia brevistyla flowers and floral scent of Camellia cultivars.Planta. 2016 Apr;243(4):959-72. doi: 10.1007/s00425-015-2454-6. Epub 2016 Jan 7. Planta. 2016. PMID: 26744017
-
Sequence-Structure Analysis Unlocking the Potential Functional Application of the Local 3D Motifs of Plant-Derived Diterpene Synthases.Biomolecules. 2024 Jan 17;14(1):120. doi: 10.3390/biom14010120. Biomolecules. 2024. PMID: 38254720 Free PMC article.
-
A pair of threonines mark ent-kaurene synthases for phytohormone biosynthesis.Phytochemistry. 2021 Apr;184:112672. doi: 10.1016/j.phytochem.2021.112672. Epub 2021 Jan 29. Phytochemistry. 2021. PMID: 33524857 Free PMC article.
-
Unraveling the Biosynthesis of Carvacrol in Different Tissues of Origanum vulgare.Int J Mol Sci. 2022 Oct 30;23(21):13231. doi: 10.3390/ijms232113231. Int J Mol Sci. 2022. PMID: 36362019 Free PMC article.
References
-
- Chan AP, Crabtree J, Zhao Q, Lorenzi H, Orvis J, Puiu D, Melake-Berhan A, Jones KM, Redman J, Chen G, Cahoon EB, Gedil M, Stanke M, Haas BJ, Wortman JR, Fraser-Liggett CM, Ravel J, Rabinowicz PD. Draft genome sequence of the oilseed species Ricinus communis. Nat Biotechnol. 2010;28:951–956. - PMC - PubMed
-
- Chen F, Tholl D, Bohlmann J, Pichersky E. The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011;66:212–229. - PubMed
-
- Christianson DW. Structural biology and chemistry of the terpenoid cyclases. Chem Rev. 2006;106:3412–3442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

