Aminoadamantanes for chronic hepatitis C
- PMID: 24793264
- PMCID: PMC10542096
- DOI: 10.1002/14651858.CD010125.pub2
Aminoadamantanes for chronic hepatitis C
Abstract
Background: Around 3% of the world's population (approximately 160 million people) are chronically infected with hepatitis C virus. The proportion of infected people who develop clinical symptoms varies between 5% and 40%. Combination therapy with pegylated interferon-alpha plus ribavirin eradicates the virus from the blood six months after treatment (sustained virological response) in approximately 40% to 80% of infected patients, depending on the viral genotype. New antiviral agents, such as boceprevir and telaprevir, in combination with standard therapy, can increase sustained virological response in genotype 1 infected patients to at least 70%. There is therefore an unmet need for drugs that can achieve a higher proportion of sustained virological response. Aminoadamantanes are antiviral drugs used for treatment of patients with chronic hepatitis C.
Objectives: To assess the beneficial and harmful effects of aminoadamantanes for patients with chronic hepatitis C infection by conducting a systematic review with meta-analyses of randomised clinical trials, as well as trial sequential analyses.
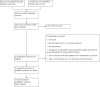
Search methods: We conducted electronic searches of the Cochrane Hepato-Biliary Group Controlled Trials Register (1996 to December 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 11 of 12 (1995 to December 2013), MEDLINE (1946 to December 2013), EMBASE (1974 to December 2013), Science Citation Index EXPANDED (1900 to December 2013), the WHO International Clinical Trials Registry Platform (www.who.int/ictrp), Google Scholar, and Eudrapharm up to December 2013 and checked the reference lists of identified publications.
Selection criteria: Randomised clinical trials assessing aminoadamantanes in patients with chronic hepatitis C infection.
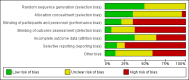
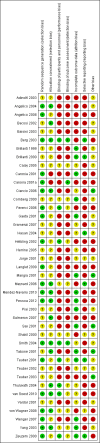
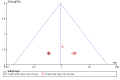
Data collection and analysis: Two authors independently extracted data. We assessed for risks of systematic errors ('bias') using the 'Risk of bias' tool. We analysed dichotomous data with risk ratio (RR) and continuous data with mean difference (MD) or standardised mean difference (SMD), both with 95% confidence intervals (CI). We used trial sequential analysis to assess the risk of random errors ('play of chance'). We assessed quality using the GRADE system.
Main results: We included 41 randomised clinical trials with 6193 patients with chronic hepatitis C. All trials had high risk of bias. All included trials compared amantadine versus placebo or no intervention. Standard antiviral therapy was administered equally to the intervention and the control groups in 40 trials. The standard antiviral therapy, which was administered to both intervention groups, was interferon-alpha, interferon-alpha plus ribavirin, and peg interferon-alpha plus ribavirin, depending on the time when the trial was conducted.When we meta-analysed all trials together, the overall results demonstrated no significant effects of amantadine, when compared with placebo or no intervention, on our all-cause mortality or liver-related morbidity composite outcome (5/2353 (0.2%) versus 6/2264 (0.3%); RR 0.90, 95% CI 0.38 to 2.17; I² = 0%; 32 trials; very low quality). There was also no significant effect on adverse events (288/2869 (10%) versus 293/2777 (11%); RR 0.98, 95% CI 0.84 to 1.14; I² = 0%; 35 trials; moderate quality). We used both fixed-effect and random-effects meta-analyses. Amantadine, when compared with placebo or no intervention, did not significantly influence the number of patients who failed to achieve a sustained virological response (1821/2861 (64%) versus 1737/2721 (64%); RR 0.98, 95% CI 0.95 to 1.02; I² = 35%; 35 trials; moderate quality). However, in the subgroup using interferon plus ribavirin, amantadine decreased the number of patients who failed to achieve a sustained virological response (422/666 (63%) versus 447/628 (71%); RR 0.89, 95% CI 0.83 to 0.96; I² = 41%; 11 trials; low quality). Similar results were found for failure to achieve an end of treatment virological response. Amantadine, when compared with placebo or no intervention, significantly decreased the number of patients without normalisation of alanine aminotransferase (ALT) serum levels at the end of treatment (671/1141 (59%) versus 732/1100 (67%); RR 0.88, 95% CI 0.83 to 0.94; I² = 47%; 19 trials; low quality). Amantadine, when compared with placebo or no intervention, did not significantly influence the end of follow-up biochemical response (1133/1896 (60%) versus 1151/1848 (62%); RR 0.95, 95% CI 0.91 to 1.00; I² = 49%; 21 trials; low quality).The observed beneficial effects could be true effects but could also be due to both systematic errors (bias) and random errors (play of chance). The latter is due to the fact that trial sequential analyses could not confirm or refute our findings. We were not able to perform meta-analyses for failure of histological improvement or quality of life due to a lack of valid data.
Authors' conclusions: This systematic review does not demonstrate any significant effects of amantadine on all-cause mortality or liver-related morbidity composite outcome and on adverse events in patients with hepatitis C; however, the median trial duration was 12 months, with a median follow-up of six months, which is not long enough to assess the composite outcome sufficiently. Overall, we did not see an effect of amantadine on failure to achieve a sustained virological response. Subgroup analyses demonstrated that the combination of amantadine plus interferon-alpha and ribavirin seems to increase the number of patients achieving a sustained virological response. This finding may be caused by both systematic errors (bias) and risks of random errors (play of chance), but it could also be real. Based on the results of the overall evidence, it appears less likely that future trials assessing amantadine for patients with chronic hepatitis C will show strong benefits. Therefore, it is probably advisable to wait for the results of trials assessing other direct-acting antiviral drugs. In the absence of convincing evidence of benefit, the use of amantadine is justified in the context of randomised clinical trials assessing the effects of combination therapy. We found a lack of evidence on other aminoadamantanes than amantadine.
Conflict of interest statement
M.H. Lamers: no declarations of interest. Mark Broekman: no declarations of interest. Joost PH Drenth: no declarations of interest. Christian Gluud: no declarations of interest.
Figures

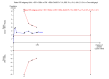


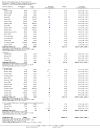
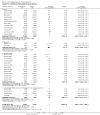

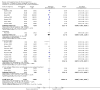
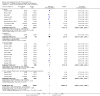
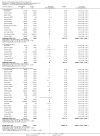

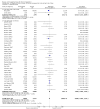
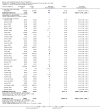
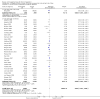





Update of
- doi: 10.1002/14651858.CD010125
Similar articles
-
Aminoadamantanes versus other antiviral drugs for chronic hepatitis C.Cochrane Database Syst Rev. 2014 Jun 17;2014(6):CD011132. doi: 10.1002/14651858.CD011132.pub2. Cochrane Database Syst Rev. 2014. PMID: 24937404 Free PMC article. Review.
-
Peginterferon plus ribavirin versus interferon plus ribavirin for chronic hepatitis C.Cochrane Database Syst Rev. 2014 Feb 28;2014(2):CD005441. doi: 10.1002/14651858.CD005441.pub3. Cochrane Database Syst Rev. 2014. PMID: 24585509 Free PMC article. Review.
-
Nitazoxanide for chronic hepatitis C.Cochrane Database Syst Rev. 2014 Apr 6;2014(4):CD009182. doi: 10.1002/14651858.CD009182.pub2. Cochrane Database Syst Rev. 2014. PMID: 24706397 Free PMC article. Review.
-
Peginterferon alpha-2a versus peginterferon alpha-2b for chronic hepatitis C.Cochrane Database Syst Rev. 2014 Feb 28;2014(2):CD005642. doi: 10.1002/14651858.CD005642.pub3. Cochrane Database Syst Rev. 2014. PMID: 24585451 Free PMC article. Review.
-
Pharmacological interventions for acute hepatitis C infection: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 13;3(3):CD011644. doi: 10.1002/14651858.CD011644.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Dec 03;12:CD011644. doi: 10.1002/14651858.CD011644.pub3. PMID: 28285495 Free PMC article. Updated. Review.
Cited by
-
Aminoadamantanes versus other antiviral drugs for chronic hepatitis C.Cochrane Database Syst Rev. 2014 Jun 17;2014(6):CD011132. doi: 10.1002/14651858.CD011132.pub2. Cochrane Database Syst Rev. 2014. PMID: 24937404 Free PMC article. Review.
References
References to studies included in this review
Adinolfi 2003 {published data only}
-
- Adinolfi LE, Marracino M, Tonziello A, Utili R, Andreana A, Giordano M, et al. Interferon induction plus ribavirin and amantadine as a novel approach for treatment of interferon nonresponder chronic hepatitis C patients. Hepatology 2000;32:352A.
-
- Adinolfi LE, Tonziello A, Utili R, Andreana A, Marracino M, Sarnataro G, et al. High response rates to interferon induction plus ribavirin and amantadine in the treatment of interferon nonresponder chronic hepatitis C patiens: a randomized controlled pilot study. Journal of Hepatology 2000;32:107.
Angelico 2004 {published data only}
-
- Angelico M, Cepparulo M, Angelico F, Barlattani A, Candilo F, Della Vecchia R, et al. A randomized controlled trial of amantadine plus interferon alpha‐2a vs. interferon alpha‐2a in naive patients with chronic hepatitis C randomized based on early response to interferon alpha‐2a alone. Hepatology 2002;36(4):589A.
-
- Angelico M, Cepparulo M, Angelico F, Francioso S, Barlattani A, Candilo F, et al. A randomized controlled trial of amantadine plus interferon‐alpha2a vs. interferon‐alpha2a alone in naive patients with chronic hepatitis C randomized according to the early virological response to interferon‐alpha2a monotherapy. Alimentary Pharmacology & Therapeutics 2004;19(3):339‐47. [PUBMED: 14984381] - PubMed
Angelico 2008 {published data only}
-
- Angelico M, Koehler‐Horst B, Piccolo P, Angelico F, Gentile S, Francioso S, et al. Final results of the SMIEC trial in naive patients with hepatitis C: double therapy (peginterferon alfa2a + ribavirin) vs peginterferon monotherapy in early viral responders and vs triple therapy (peginterferon + ribavirin + amantadine) in early non responders. Hepatology 2005;42:696A. - PubMed
-
- Angelico M, Koehler‐Horst B, Piccolo P, Angelico F, Gentile S, Francioso S, et al. Peginterferon alpha‐2a and ribavirin versus peginterferon alpha‐2a monotherapy in early virological responders and peginterferon alpha‐2a and ribavirin versus peginterferon alpha‐2a, ribavirin and amantadine triple therapy in early virological nonresponders: the SMIEC II trial in naive patients with chronic hepatitis C. European Journal of Gastroenterology & Hepatology 2008;20(7):680‐7. [PUBMED: 18679072] - PubMed
Bacosi 2002 {published data only}
-
- Bacosi M, Russo F, D'innocenzo S, Santolamazza M, Miglioresi L, Ursitti A, et al. Amantadine and interferon in the combined treatment of hepatitis C virus in elderly patients. Hepatology Research 2002;22(3):231‐9. [PUBMED: 11882420] - PubMed
Baisini 2003 {published data only}
-
- Baisini O, Gracielle Pigozzi M, Benini F, Stellini R, Reggiani A, Quattrocchi D, et al. A randomised, open label, controlled trial on the effect of interferon plus amantadine compared with interferon alone for treatment of chronic hepatitis C. Hepatology Research 2003;26(3):167‐73. [PUBMED: 12850687] - PubMed
-
- Gracielle Pigozzi M, Stellini R, Reggiani A, Baisini O, Salmi A, Andri G, et al. Interferon (IFN) vs interferon + amantadine hydrochloride (IFN+A) for treatment of chronic hepatitis C: an ongoing multicentre randomized trial. Gastroenterology 2000;118(4):A1480.
-
- Lanzini A, Pigozzo MG, Stellini R, Reggiani A, Salmi A, Andri A, et al. Interferon (IFN) vs interferon + amantadine (IFN+A) for treatment of chronic hepatitis C: results of a randomised controlled trial. Hepatology 2001;34(4):338A.
Berg 2003 {published data only}
-
- Berg T, Kronenberger B, Hinrichsen H, Gerlach T, Buggisch P, Herrmann E, et al. Triple therapy with amantadine in treatment‐naive patients with chronic hepatitis C: a placebo‐controlled trial. Hepatology 2003;37(6):1359‐67. [PUBMED: 12774015] - PubMed
-
- Berg T, Kronenberger B, Hinrichsen H, Gerlach T, Buggisch P, Spengler U, et al. Triple therapy with amantadine sulphate plus ribavirin and interferon‐alpha in previously untreated patients with chronic hepatitis C: results of a double‐blind, placebo‐controlled trial. Journal of Hepatology 2002;36:3.
-
- Berg T, Kronenberger, B, Hinrichsen H, Gerlach T, Buggisch P, Spengler U, et al. Randomized, double‐blind, placebo‐controlled trial of high‐dose interferon‐alfa induction combination therapy with and without amantadine sulphate in previously untreated patients with chronic hepatitis C. Hepatology 2001;34:330A.
-
- Kronenberger B, Berg T, Herrmann E, Hinrichsen H, Gerlach T, Buggisch P, et al. Efficacy of amantadine on quality of life in patients with chronic hepatitis C treated with interferon‐α and ribavirin: results from a randomized, placebo‐controlled, double‐blind trial. European Journal of Gastroenterology & Hepatology 2007;19:639‐46. - PubMed
Brillanti 1999 {published data only}
-
- Brillanti S, Foli M, Tomaso M, Gramantieri L, Masci C, Bolondi L. Pilot study of triple antiviral therapy for chronic hepatitis C in interferon alpha non‐responders. Italian Journal of Gastroenterology and Hepatology 1999;31(2):130‐4. [PUBMED: 10363198] - PubMed
Brillanti 2000 {published data only}
-
- Brillanti S, Levantesi F, Masi L, Foli M, Bolondi L. Triple antiviral therapy as a new option for patients with interferon nonresponsive chronic hepatitis C. Hepatology 2000;32(3):630‐4. [PUBMED: 10960460] - PubMed
Calay 2005 {published data only}
-
- Calay V, Hachemane S, Pageaux GP, Bourliere M, Portal I, Mathurin P, et al. Does amantadine improve the virological sustained response of naive patients with chronic hepatitis C in association with peg‐interferon‐ribavirin combination? A prospective, randomised, multicenter, double‐blind study: preliminary results in 200 patients. Hepatology 2005;44:704A‐5A.
-
- Calay V, Hachemane S, Pageaux GP, Canva M, Bourliere M, Portal I, et al. A multicentric, randomized, controlled, double‐blind study (TRIPEG): PEGINF α‐2b, ribavirin and amantadine or PEGINF α‐2b, ribavirin and placebo, in 269 naive patients with chronic hepatitis C. Journal of Hepatology 2006;44:S220.
Caronia 2001 {published data only}
-
- Caronia S, Bassendine MF, Barry R, Mills P, Naoumov NV, Fox R, et al. Interferon plus amantadine versus interferon alone in the treatment of naive patients with chronic hepatitis C: a UK multicentre study. Journal of Hepatology 2001;35(4):512‐6. [PUBMED: 11682036] - PubMed
-
- Caronia S, Bassendine MF, Mills P, Naumov N, Fox R, Lowes R, et al. Interferon plus amantadine versus interferon alone in the treatment of naive patients with chronic hepatitis C: a UK multicentre study. Journal of Hepatology 2000;32:110. - PubMed
-
- Caronia S, Crossey M, Bassendine M, Mills P, Fox R, Naoumov N, et al. Interferon plus amantadine versus interferon alone in the treatment of naive patients with chronic hepatitis C: interim analysis of multicentre study. Hepatology 1999;30:628A. - PubMed
-
- Caronia S, Crossey M, Murray‐Lyon I, Lypsnyi D, Main J, Thomas HC, et al. A pilot study of interferon plus amantadine versus interferon alone in the treatment of chronic hepatitis C. Journal of Hepatology 1999;30:138. - PubMed
Caronia 2001a {published data only}
-
- Caronia S, Bassendine MF, Barry R, Mills P, Naoumov NV, Fox R, et al. Interferon plus amantadine versus interferon alone in the treatment of naive patients with chronic hepatitis C: a UK multicentre study. Journal of Hepatology 2001;35(4):512‐6. [PUBMED: 11682036] - PubMed
Ciancio 2006 {published data only}
-
- Ciancio A, Picciotto A, Giordanino C, Smedile A, Tabone M, Manca A, et al. A randomised trial of pegylated interferon alpha‐2a plus ribavirin with or without amantadine in the re‐treatment of patients with chronic hepatitis C not responding to standard interferon and ribavirin. Journal of Clinical Virology 2006;36:S148. - PubMed
-
- Ciancio A, Picciotto A, Giordanino C, Smedile A, Tabone M, Manca A, et al. A randomized trial of pegylated‐interferon‐alpha2a plus ribavirin with or without amantadine in the re‐treatment of patients with chronic hepatitis C not responding to standard interferon and ribavirin. Alimentary Pharmacology & Therapeutics 2006;24(7):1079‐86. [PUBMED: 16984502] - PubMed
Cornberg 2000 {published data only}
-
- Cornberg M, Jaeckel M, Schueler A, Koerbel JN, Wedemeyer H, Mansuroglu T, et al. Treatment of chronic hepatitis C in nonresponder patients with high daily induction dosing of interferon‐alfa, ribavirin and amantadine/placebo. Hepatology 2000;21:351A.
Ferenci 2006 {published data only}
-
- Ferenci P, Formann E, Gschwantler M, Laferl H, Brunner H, Hackl F, et al. Prospective evaluation of the 24 hour interferon (IFN) induced decline in hepatitis C virus genotype 1 load to predict sustained virologic response (SVR) to peginterferon‐alfa2a/ribavirin combination therapy with or without amantadine. Journal of Hepatology 2004;40:141.
-
- Ferenci P, Formann E, Laferl H, Gschwantler M, Hackl F, Brunner H, et al. Randomized, controlled, double‐blind, placebo‐controlled study of peginterferon alfa‐2a (40KD) (Pegasys®) plus ribavirin (Copegus®) and amantadine (AMA) or placebo in patients with chronic hepatitis C genotype 1 infection. Hepatology 2004;40:396A. - PubMed
-
- Ferenci P, Formann E, Laferl H, Gschwantler M, Hackl F, Brunner H, et al. Randomized, double‐blind, placebo‐controlled study of peginterferon alfa‐2a (40KD) plus ribavirin with or without amantadine in treatment‐naive patients with chronic hepatitis C genotype 1 infection. Journal of Hepatology 2006;44(2):275‐82. [PUBMED: 16338019] - PubMed
-
- Formann E, Jessner W, Hubmann R, Datz C, Munda‐Steindl P, Klingler A, et al. Effect of amantadine on quality of life and fatigue in patients with chronic hepatitis C participating in a prospective, double blind, placebo controlled, randomized trial of treatment with pegylated interferon alfa2a/ribavirin. Hepatology 2004;40:338A.
Gaeta 2001 {published data only}
-
- Gaeta GB, Stornaiuolo G, Ascione T, Stanzione M, Pasquazzi C, Cimono L, et al. Interferon alfa‐2a (IFN) plus amantadine in chronic hepatitis C resistant to IFN‐α alone: a pilot randomised study. Hepatology 1999;30:632A. - PubMed
-
- Gaeta GB, Stornaiuolo G, Stanzione M, Ascione T, Pasquazzi C, Taliani G, et al. Interferon‐alpha plus amantadine in chronic hepatitis C resistant to interferon alone: a pilot randomized study. Journal of Viral Hepatitis 2001;8(4):284‐6. [PUBMED: 11454180] - PubMed
Gramenzi 2007 {published data only}
-
- Andreone P, Gramenzi A, Cursaro C, Pirraglia C, Verrucchi G, Attard L, et al. IFN plus ribavirin and amantadine for IFN‐sensitive patients with chronic hepatitis C: results of a multicenter, randomized, controlled trial. Journal of Hepatology 2003;38(S2):124.
-
- Gramenzi A, Andreone P, Cursaro C, Verucchi G, Boccia S, Giacomoni PL, et al. A randomized trial of induction doses of interferon alone or in combination with ribavirin or ribavirin plus amantadine for treatment of nonresponder patients with chronic hepatitis C. Journal of Gastroenterology 2007;42(5):362‐7. [PUBMED: 17530360] - PubMed
Hasan 2004 {published data only}
-
- Hasan F, Al‐Khaldi J, Asker H, Al‐Ajmi M, Owayed S, Varghese R, et al. Peginterferon alpha‐2b plus ribavirin with or without amantadine [correction of amantidine] for the treatment of non‐responders to standard interferon and ribavirin. Antiviral Therapy 2004;9(4):499‐503. [PUBMED: 15456080] - PubMed
Helbling 2002 {published data only}
-
- Helbling B, Hermann R, Stamenic I, Viani F, Gonvers JJ, Dufour JF, et al. Interferon alfa‐2a (IFN) and amantadine (A) or placebo (P) in IFN‐naive patients with chronic hepatitis C (CHC): a double‐blind, randomized, placebo‐controlled trial. Hepatology 2001;34(4):331A. - PubMed
-
- Helbling B, Stamenic I, Viani F, Gonvers JJ, Dufour JF, Reichen J, et al. Interferon and amantadine in naive chronic hepatitis C: a double‐blind, randomized, placebo‐controlled trial. Hepatology 2002;35(2):447‐54. [PUBMED: 11826422] - PubMed
Herrine 2005 {published data only}
-
- Herrine SK, Brown RS Jr, Bernstein DE, Ondovik MS, Lentz E, Te H. Peginterferon alpha‐2a combination therapies in chronic hepatitis C patients who relapsed after or had a viral breakthrough on therapy with standard interferon alpha‐2b plus ribavirin: a pilot study of efficacy and safety. Digestive Diseases and Sciences 2005;50(4):719‐26. [PUBMED: 15844708] - PubMed
Jorge 2001 {published data only}
-
- Jorge FA, Tanno H, Igartua EB, Pinchuk L, Sorda JA, Curciarello J, et al. Interferon induction treatment with and without amantadine HCl (AHCl) in patients with chronic hepatitis C. Journal of Hepatology 2001;34:156‐7.
Langlet 2009 {published data only}
-
- Langlet P, D'Heygere F, Henrion J, Adler M, Delwaide J, Vlierberghe H, et al. Clinical trial: a randomized trial of pegylated‐interferon‐alpha‐2a plus ribavirin with or without amantadine in treatment‐naive or relapsing chronic hepatitis C patients. Alimentary Pharmacology & Therapeutics 2009;30(4):352‐63. [PUBMED: 19485978] - PubMed
Mangia 2001 {published data only}
-
- Mangia A, Minerva N, Annese M, Leandro G, Villani MR, Santoro R, et al. A randomized trial of amantadine and interferon versus interferon alone as initial treatment for chronic hepatitis C. Hepatology 2001;33(4):989‐93. [PUBMED: 11283865] - PubMed
-
- Mangia A, Santoro R, Villani MR, Soccio T, Cela O, Annese M, et al. Long term response to amantadine + interferon is significantly higher than that to interferon alone in chronic hepatitis C: final report of a randomized clinical trial. Hepatology 2000;32:318A.
Maynard 2006 {published data only}
-
- Maynard M, Ahmed SNS, Bailly F, Rozier F, Benmakhlouf N, Bourne‐Branchu V. Retreatment of IFN/ribavirin non‐responder hepatitis C patients: benefit of peg‐interferon/ribavirin/amantadine. Hepatology 2004;40:398A.
-
- Maynard M, Pradat P, Bailly F, Rozier F, Nemoz C, Si Ahmed SN, et al. Amantadine triple therapy for non‐responder hepatitis C patients. Clues for controversies (ANRS HC 03 BITRI). Journal of Hepatology 2006;44(3):484‐90. [PUBMED: 16426697] - PubMed
Mendez‐Navarro 2010 {published data only}
-
- Mendez‐Navarro J, Chirino RA, Corey KE, Gorospe EC, Zheng H, Chung R, et al. Assessment and prediction of sustained virological response among treatment‐naive Latinos with, genotype 1 HCV treated with standard double versus triple therapy with amantadine. Hepatology 2009;50:707A.
Pessoa 2012 {published data only}
-
- Cheinquer H, Pessoa M, Almeida P, Silva G, Patelli M, Parana R, et al. Prospective randomized study of peginterferon alpha‐2a plus ribavirin, in hepatitis C patients non‐responders or relapsers to interferon‐alpha and ribavirin. Journal of Hepatology 2006;44:S209.
-
- Pessoa MG, Cheinquer H, Almeida PR, Silva GF, Lima MP, Parana R, et al. Re‐treatment of previous non‐responders and relapsers to interferon plus ribavirin with peginterferon alfa‐2a (40KD), ribavirin +/‐ amantadine in patients with chronic hepatitis C: randomized multicentre clinical trial. Annals of Hepatology 2012;11(1):52‐61. [PUBMED: 22166561] - PubMed
Piai 2003 {published data only}
-
- Piai G, Rocco P, Tartaglione MT, Ciarleglio A, Focareta R, Grimaldi E, et al. Triple (interferon, ribavirin, amantadine) versus double (interferon, ribavirin) re‐therapy for interferon relapser genotype 1b HCV chronic active hepatitis patients. Hepatology Research 2003;25(4):355‐63. [PUBMED: 12699845] - PubMed
Salmeron 2007 {published data only}
-
- Salmeron J, Diago M, Andrade R, Perez R, Sola R, Romero M, et al. Induction doses of interferon‐alpha‐2a in combination with ribavirin and/or amantadine for the treatment of chronic hepatitis C in non‐responders to interferon monotherapy: a randomized trial. Journal of Viral Hepatitis 2007;14(2):89‐95. [PUBMED: 17244248] - PubMed
Sax 2001 {published data only}
-
- Sax H, Friedl A, Renner E, Steuerwald MH, Weber R. Pilot study of interferon‐alpha with and without amantadine for the treatment of hepatitis C in HIV coinfected individuals on antiretroviral therapy. Infection 2001;29(5):267‐70. [PUBMED: 11688904] - PubMed
Shakil 2000 {published data only}
-
- Ahmad J, Vargas H, Balan V, Rakela J, Shakil AO. A randomized, double‐blind, placebo‐controlled trial of interferon‐alpha and amantadine versus interferon‐alpha alone in the treatment of patients with chronic hepatitis C. Digestive Diseases and Sciences 2002;47(7):1655‐6. - PubMed
-
- Shakil AO, Balan V, Vargas H, Gartner K, Rakela J. A randomized, double‐blind, placebo controlled trial of interferon‐alfa and amantadine versus interferon‐alfa alone in the treatment of chronic hepatitis C. Gastroenterology 2000;118:A1487. - PubMed
Smith 2004 {published data only}
-
- Smith JP, Riley TR 3rd, Devenyi A, Bingaman SI, Kunselman A. Amantadine therapy for chronic hepatitis C: a randomized double‐blind placebo controlled trial. Gastroenterology 2002;122(S4):A‐627.
Tabone 2001 {published data only}
-
- Tabone M, Delmastro B, Bonardi R, Andreoni M, Manca A, Calleri G, et al. Combination therapy interferon plus amantadine in chronic active hepatitis C. A multicentric randomised study: preliminary reports. Hepatology 1999;30:628A.
-
- Tabone M, Laudi C, Delmastro B, Biglino A, Andreoni M, Chieppa F, et al. Interferon and amantadine in combination as initial treatment for chronic hepatitis C patients. Journal of Hepatology 2001;35(4):517‐21. [PUBMED: 11682037] - PubMed
Teuber 2001 {published data only}
-
- Teuber G, Berg T, Naumann U, Raedle J, Brinkmann S, Hopf U, et al. Randomized, placebo‐controlled, double‐blind trial with interferon‐alpha with and without amantadine sulphate in primary interferon‐alpha nonresponders with chronic hepatitis C. Journal of Viral Hepatitis 2001;8(4):276‐83. [PUBMED: 11454179] - PMC - PubMed
-
- Teuber G, Naumann U, Berg T, Dreher A, Hopf U, Zeuzem S. Randomized, placebo‐controlled, double‐blind trial with interferon‐A (IFN A) with and without amantadin‐sulfat in primary IFN‐A non‐responders with chronic hepatitis C. Hepatology 2000;32:354A.
-
- Teuber G, Naumann U, Berg T, Raedle J, Dreher A, Hopf U, et al. Multicenter, randomized, double‐blind, placebo‐controlled trial of interferon‐A (IFN‐A2A) with and without amantadine as treatment for IFN‐A non‐responders with chronic hepatitis C. Gastroenterology 2000;118:A1494.
Teuber 2002 {published data only}
-
- Teuber G, Pascu M, Lafrenz M, Weidenbach H, Schoelmrich J, Wietske‐Braun P, et al. Randomized, controlled trial with interferon‐alfa plus ribavirin with and without amantadine sulphate in patients with chronic hepatitis C relapsing after primary successful antiviral treatment. Hepatology 2001;34:579A.
-
- Teuber G, Pascu M, Lafrenz M, Weidenbach H, Schoelmrich J, Wietske‐Braun P, et al. Randomized, controlled trial with interferon‐alpha plus ribavirin with and without amantadine sulphate in patients with chronic hepatitis C relapsing after previous successful antiviral treatment. Journal of Hepatology 2002;36:132.
Teuber 2003 {published data only}
-
- Teuber G, Berg T, Lafrenz M, Weidenbach H, Schoelmerich J, Wietzke‐Braun P, et al. Randomized, controlled trial with interferon‐alpha (IFN‐alpha) combined with ribavirin with and without amantadine sulfate in primary IFN‐alpha nonresponsive patients with chronic hepatitis C. Journal of Hepatology 2001;34(1):23.
-
- Teuber G, Berg T, Lafrenz M, Weidenbach H, Schoelmerich J, Wietzke‐Braun P, et al. Randomized, controlled trial with interferon‐α (IFN‐α) combined with ribavirin with and without amantadinsulfat in primary IFN‐α non‐responders with chronic hepatitis C. Hepatology 2001;120:A381.
-
- Teuber G, Pascu M, Berg T, Lafrenz M, Pausch J, Kullmann F, et al. Randomized, controlled trial with IFN‐alpha combined with ribavirin with and without amantadine sulphate in non‐responders with chronic hepatitis C. Journal of Hepatology 2003;39(4):606‐13. [PUBMED: 12971972] - PubMed
Thuluvath 2004 {published data only}
-
- Thuluvath PJ, Maheshwari A, Mehdi J, Fairbanks KD, Wu LL, Gelrud LG, et al. Randomised, double blind, placebo controlled trial of interferon, ribavirin, and amantadine versus interferon, ribavirin, and placebo in treatment naive patients with chronic hepatitis C. Gut 2004;53(1):130‐5. [PUBMED: 14684587] - PMC - PubMed
van Soest 2010 {published data only}
-
- Soest H, Schaar PJ, Koek GH, Vries RA, Ooteghem NA, Hoek B, et al. No beneficial effects of amantadine in treatment of chronic hepatitis C patients. Digestive and Liver Disease 2010;42(7):496‐502. [PUBMED: 20018575] - PubMed
Vardar 2001 {published data only}
-
- Vardar R, Karasu Z, Gunsar F, Akarca U, Ersoz G, Batur Y. Interferon plus amantadine has no superiority to IFN monotherapy in naive patients with chronic hepatitis C. Journal of Hepatology 2001;34:178.
von Wagner 2008 {published data only}
-
- Wagner M, Hofmann WP, Teuber G, Berg T, Goeser T, Spengler U, et al. Placebo‐controlled trial of 400 mg amantadine combined with peginterferon alfa‐2a and ribavirin for 48 weeks in chronic hepatitis C virus‐1 infection. Hepatology 2008;48(5):1404‐11. [PUBMED: 18846541] - PubMed
-
- Wagner M, Hofmann WP, Teuber G, Berg T, Goeser T, Spengler U, et al. Randomized, double‐blind, placebo‐controlled trial of peginterferon alfa‐2a (40KD) and ribavirin with and without 400 mg amantadine‐sulphate for 48 weeks in treatment naive HCV genotype 1‐infected patients. Hepatology 2007;46:342A‐3A.
-
- Wagner M, Hofmann WP, Teuber G, Berg T, Goeser T, Spengler U, et al. Sustained virologic response is associated with worse QoL during and improved QoL after treatment with peginterferon alfa‐2a and ribavirin. Hepatology 2008;48:886A‐7A. - PubMed
Wenger 2007 {published data only}
-
- Wenger C, Bischof T, Gonvers JJ, Renner EL, Mullhaupt B. Interferon and ribavirin with or without amantadine for interferon non‐responders with chronic hepatitis C. A randomized, controlled pilot study. Swiss Medical Weekly 2007;137(29‐30):418‐23. [PUBMED: 17705104] - PubMed
Yang 2003 {published data only}
-
- Yang SS, Tu TC, Wu CH, Chen DS. Combination of interferon alfa‐2a and amantadine does not improve the efficacy of interferon therapy in patients with chronic hepatitis C. Hepato‐Gastroenterology 2003;50(53):1575‐8. [PUBMED: 14571789] - PubMed
-
- Yang SS, Tu TC, Wu CH, Chen DS. Combination of interferon alfa‐2a and amantadine does not improve the efficacy of interferon therapy in patients with chronic hepatitis C. Hepatology 1999;30:369A. - PubMed
Zeuzem 2000 {published data only}
References to studies excluded from this review
Buggisch 2009 {published data only}
-
- Buggisch P, Zeuzem S, Teuber G, Möller B, Ehrhardt A, Gelbmann C, et al. Treating relapse with peginterferon alfa‐2a plus ribavirin and amantadin for either 72 or 48 weeks (in patients with HDV‐type 1/3 infection): results from the TRELA‐study. Journal of Hepatology 2009;50(S1):S382.
Di Bisceglie 2001 {published data only}
-
- Bisceglie AM, Bernstein DE, Rustgi VR, Gitlin N, Jeffers LJ, Simon D, et al. Pegylated (40 KDA) interferon alfa‐2a (Pegasys®) in new combination therapies: a report of a randomized, multicenter efficacy and safety study. Journal of Hepatology 2001;34:143.
Mendez‐Navarro 2010a {published data only}
Nakamura 2003 {published data only}
-
- Nakamura H, Uyama H, Enomoto H, Kishima Y, Yamamoto M, Yoshida K, et al. The combination therapy of interferon and amantadine hydrochloride for patients with chronic hepatitis C. Hepato‐Gastroenterology 2003;50(49):222‐6. [PUBMED: 12630027] - PubMed
Popovic 2000 {published data only}
-
- Popovic A, Vian A, Lorenzoni U, Lobello S, Pevere S, Silverij E, et al. Daily interferon monotherapy versus daily interferon plus amantadine in the treatment of naive chronic hepatitis C patients: short‐term response. Journal of Hepatology. 2000; Vol. 32:195.
Quarantini 2006 {published data only}
-
- Quarantini LC, Miranda‐Scippa A, Schinoni MI, Sampaio AS, Santos‐Jesus R, Bressan RA, et al. Effect of amantadine on depressive symptoms in chronic hepatitis C patients treated with pegylated interferon: a randomized, controlled pilot study. Clinical Neuropharmacology 2006;29(3):138‐43. [PUBMED: 16772812] - PubMed
Schories 2003 {published data only}
-
- Schories M, Kallinowski B, Goeser T, Maier KP, Lutterer A, Wietzke‐Braun V, et al. Randomized placebo controlled double blind study in patients with chronic hepatitis C who did not respond to interferon therapy: comparison between interferon A, ribavirin +/‐ amantadine. Journal of Hepatology 2003;38:164.
-
- Schories M, Kallinowski B, Goeser T, Maier KP, Wietzke‐Braun V, Lutterer A, et al. Randomized placebo controlled double blind study in interferon nonresponder chronic hepatitis C patients with interferon A, ribavirin, amantadine in comparison to interferon A and ribavirin. Hepatology 2002;36(4):572A.
Torre 1999 {published data only}
-
- Torre F, Brizzolara R, Giusto R, Grasso A, Sinelli N, Campo N, et al. HCV‐RNA dynamics in the first month of treatment with IFN daily therapy alone versus combinations with amantadine or ribavirin. Journal of Hepatology 1999;30:128.
Zilly 2002 {published data only}
-
- Zilly M, Lingenauber C, Desch S, Vath T, Klinker H, Langmann P. Triple antiviral re‐therapy for chronic hepatitis C with interferon‐alpha, ribavirin and amantadine in nonresponders to interferon‐alpha and ribavirin. European Journal of Medical Research 2002;7(4):149‐54. [PUBMED: 12010649] - PubMed
Additional references
Altman 2003
Alves Galvão 2012
Asselah 2010
Awad 2010
-
- Awad T, Thorlund K, Hauser G, Stimac D, Mabrouk M, Gluud C. Peginterferon alpha‐2a is associated with higher sustained virological response than peginterferon alfa‐2b in chronic hepatitis C: systematic review of randomized trials. Hepatology 2010;51(4):1176‐84. [PUBMED: 20187106] - PubMed
Bacon 2011
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. - PubMed
Brok 2009
Brok 2009a
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
Brok 2010
Chen 2012
-
- Chen J, Shi J, Xie WF, Zeng X, Lin Y. Meta‐analysis: amantadine may lower the efficacy of pegylated interferon plus ribavirin in treatment‐naive hepatitis C genotype 1 patients. International Journal of Infectious Diseases 2012;16(10):e748‐52. [PUBMED: 22836046] - PubMed
Choo 1989
-
- Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood‐borne non‐A, non‐B viral hepatitis genome. Science 1989;244(4902):359‐62. [PUBMED: 2523562] - PubMed
Chutaputti 2000
-
- Chutaputti A. Adverse effects and other safety aspects of the hepatitis C antivirals. Journal of Gastroenterology and Hepatology 2000;15(Suppl):E156‐63. [PUBMED: 10921400] - PubMed
Crosby 2009
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. ctu.dk/tsa/ 2011 (accessed 4 September 2013).
Davis 1989
-
- Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC Jr, Perrillo RP, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. New England Journal of Medicine 1989;321(22):1501‐6. [PUBMED: 2509916] - PubMed
Davis 1998
-
- Davis GL, Esteban‐Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, et al. Interferon alfa‐2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. New England Journal of Medicine 1998;339(21):1493‐9. [PUBMED: 9819447] - PubMed
De Clercq 2001
-
- Clercq E. Antiviral drugs: current state of the art. Journal of Clinical Virology 2001;22(1):73‐89. [PUBMED: 11418355] - PubMed
Deltenre 2004
-
- Deltenre P, Henrion J, Canva V, Dharancy S, Texier F, Louvet A, et al. Evaluation of amantadine in chronic hepatitis C: a meta‐analysis. Journal of Hepatology 2004;41(3):462‐73. [PUBMED: 15336450] - PubMed
DeMets 1987
-
- DeMets DL. Methods for combining randomised clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [PUBMED: 3616287] - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] - PubMed
Di Bisceglie 2011
Di Martino 2011
-
- Martino V, Crouzet J, Hillon P, Thevenot T, Minello A, Monnet E. Long‐term outcome of chronic hepatitis C in a population‐based cohort and impact of antiviral therapy: a propensity‐adjusted analysis. Journal of Viral Hepatitis 2011;18(7):493‐505. [PUBMED: 21692956] - PubMed
EASL 2011
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. Journal of Hepatology 2011;55(2):245‐64. [PUBMED: 21371579] - PubMed
Egger 1997
Egger 2003
-
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technology Assessment 2003;7(1):1‐76. [PUBMED: 12583822] - PubMed
El‐Serag 2003
-
- El‐Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Annals of Internal Medicine 2003;139(10):817‐23. [PUBMED: 14623619] - PubMed
Ghany 2009
Gluud 1998
-
- Gluud C, Nikolova D. Quality assessment of reports on clinical trials in the Journal of Hepatology. Journal of Hepatology 1998;29(2):321‐7. [PUBMED: 9722217] - PubMed
Gluud 2007
-
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. - PubMed
Gluud 2014
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2014, Issue 1. Art. No.: LIVER.
Gurusamy 2013
-
- Gurusamy KS, Wilson E, Koretz RL, Allen VB, Davidson BR, Burroughs AK, et al. Is sustained virological response a marker of treatment efficacy in patients with chronic hepatitis C viral infection with no response or relapse to previous antiviral intervention?. PloS One 2013;8(12):e83313. [PUBMED: 24349487] - PMC - PubMed
Guyatt 2008
Hauser 2014
Hauser 2014a
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollis 1999
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice 1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Jacobson 2011
-
- Jacobson IM, McHutchison JG, Dusheiko G, Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. New England Journal of Medicine 2011;364(25):2405‐16. [PUBMED: 21696307] - PubMed
Jefferson 2012
Kenny‐Walsh 1999
-
- Kenny‐Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti‐D immune globulin. Irish Hepatology Research Group. New England Journal of Medicine 1999;340(16):1228‐33. [PUBMED: 10210705] - PubMed
Kim 2009
Kjaergard 1999
-
- Kjaergard LL, Nikolova D, Gluud C. Randomized clinical trials in HEPATOLOGY: predictors of quality. Hepatology 1999;30(5):1134‐8. [PUBMED: 10534332] - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomised trials in meta‐analyses. Annals of Internal Medicine 2001;135:982‐9. - PubMed
Koff 1980
Koretz 1993
-
- Koretz RL, Abbey H, Coleman E, Gitnick G. Non‐A, non‐B post‐transfusion hepatitis. Looking back in the second decade. Annals of Internal Medicine 1993;119(2):110‐5. [PUBMED: 8512159] - PubMed
Koretz 2013
Lauer 2001
-
- Lauer GM, Walker BD. Hepatitis C virus infection. New England Journal of Medicine 2001;345(1):41‐52. [PUBMED: 11439948] - PubMed
Lavanchy 2011
-
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clinical Microbiology and Infection 2011;17(2):107‐15. [PUBMED: 21091831] - PubMed
Lim 2005
Lundh 2012
McHutchison 1998
-
- McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa‐2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. New England Journal of Medicine 1998;339(21):1485‐92. [PUBMED: 9819446] - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. - PubMed
Myers 2002
Needleman 1999
-
- Needleman I. CONSORT. Consolidated Standards of Reporting Trials. British Dental Journal 1999;186(5):207. - PubMed
Poordad 2011
Poynard 1998
-
- Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 1998;352(9138):1426‐32. [PUBMED: 9807989] - PubMed
Reichard 1991
-
- Reichard O, Andersson J, Schvarcz R, Weiland O. Ribavirin treatment for chronic hepatitis C. Lancet 1991;337(8749):1058‐61. [PUBMED: 1673493] - PubMed
Reichard 1993
-
- Reichard O, Yun ZB, Sonnerborg A, Weiland O. Hepatitis C viral RNA titers in serum prior to, during, and after oral treatment with ribavirin for chronic hepatitis C. Journal of Medical Virology 1993;41(2):99‐102. [PUBMED: 8283183] - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rodger 2000
-
- Rodger AJ, Roberts S, Lanigan A, Bowden S, Brown T, Crofts N. Assessment of long‐term outcomes of community‐acquired hepatitis C infection in a cohort with sera stored from 1971 to 1975. Hepatology 2000;32(3):582‐7. [PUBMED: 10960453] - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomised controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Savović 2012
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Schultz 1995
-
- Schultz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Schulz 2012
-
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Internal Journal of Surgery 2011;9(8):672‐7. - PubMed
Seeff 2009
Sherman 2011
Simin 2007
-
- Simin M, Brok J, Stimac D, Gluud C, Gluud LL. Cochrane systematic review: pegylated interferon plus ribavirin vs. interferon plus ribavirin for chronic hepatitis C. Alimentary Pharmacology & Therapeutics 2007;25(10):1153‐62. [PUBMED: 17451561] - PubMed
Simmonds 2005
-
- Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, Feinstone S, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 2005;42(4):962‐73. [PUBMED: 16149085] - PubMed
Smith 2004a
-
- Smith JP, Riley TR 3rd, Bingaman S, Mauger DT. Amantadine therapy for chronic hepatitis C: a dose escalation study. American Journal of Gastroenterology 2004;99(6):1099‐104. [PUBMED: 15180732] - PubMed
Soza 2002
-
- Soza A, Everhart JE, Ghany MG, Doo E, Heller T, Promrat K, et al. Neutropenia during combination therapy of interferon alfa and ribavirin for chronic hepatitis C. Hepatology 2002;36(5):1273‐9. [PUBMED: 12395340] - PubMed
SPIRIT 2013
SPIRIT 2013a
Sulkowski 2004
-
- Sulkowski MS, Wasserman R, Brooks L, Ball L, Gish R. Changes in haemoglobin during interferon alpha‐2b plus ribavirin combination therapy for chronic hepatitis C virus infection. Journal of Viral Hepatitis 2004;11(3):243‐50. [PUBMED: 15117326] - PubMed
Sy 2006
Thein 2008
-
- Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage‐specific fibrosis progression rates in chronic hepatitis C virus infection: a meta‐analysis and meta‐regression. Hepatology 2008;48(2):418‐31. [PUBMED: 18563841] - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 23 September 2013).
Tine 1991
-
- Tine F, Magrin S, Craxi A, Pagliaro L. Interferon for non‐A, non‐B chronic hepatitis. A meta‐analysis of randomised clinical trials. Journal of Hepatology 1991;13(2):192‐9. [PUBMED: 1835989] - PubMed
Ueno 2009
-
- Ueno Y, Sollano JD, Farrell GC. Prevention of hepatocellular carcinoma complicating chronic hepatitis C. Journal of Gastroenterology and Hepatology 2009;24(4):531‐6. [PUBMED: 19368633] - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Wiese 2000
-
- Wiese M, Berr F, Lafrenz M, Porst H, Oesen U. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single‐source outbreak in Germany: a 20‐year multicenter study. Hepatology 2000;32(1):91‐6. [PUBMED: 10869294] - PubMed
Wood 2008
Zeuzem 2011
-
- Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. New England Journal of Medicine 2011;364(25):2417‐28. [PUBMED: 21696308] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

