Uterine NK cells: active regulators at the maternal-fetal interface
- PMID: 24789879
- PMCID: PMC4001528
- DOI: 10.1172/JCI68107
Uterine NK cells: active regulators at the maternal-fetal interface
Abstract
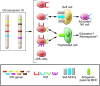
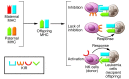
Pregnancy presents an immunological conundrum because two genetically different individuals coexist. The maternal lymphocytes at the uterine maternal-fetal interface that can recognize mismatched placental cells are T cells and abundant distinctive uterine NK (uNK) cells. Multiple mechanisms exist that avoid damaging T cell responses to the fetus, whereas activation of uNK cells is probably physiological. Indeed, genetic epidemiological data suggest that the variability of NK cell receptors and their MHC ligands define pregnancy success; however, exactly how uNK cells function in normal and pathological pregnancy is still unclear, and any therapies aimed at suppressing NK cells must be viewed with caution. Allorecognition of fetal placental cells by uNK cells is emerging as the key maternal-fetal immune mechanism that regulates placentation.
Figures

Similar articles
-
Maternal KIR and fetal HLA-C: a fine balance.J Leukoc Biol. 2011 Oct;90(4):703-16. doi: 10.1189/jlb.0511227. Epub 2011 Aug 26. J Leukoc Biol. 2011. PMID: 21873457 Review.
-
Natural killer cells and pregnancy.Nat Rev Immunol. 2002 Sep;2(9):656-63. doi: 10.1038/nri886. Nat Rev Immunol. 2002. PMID: 12209134 Review.
-
Emerging role of uterine natural killer cells in establishing pregnancy.Iran J Immunol. 2008 Jun;5(2):71-81. Iran J Immunol. 2008. PMID: 18523352 Review.
-
Uterine Natural Killer Cells.Front Immunol. 2019 May 1;10:960. doi: 10.3389/fimmu.2019.00960. eCollection 2019. Front Immunol. 2019. PMID: 31118936 Free PMC article. Review.
-
A New Look at Immunogenetics of Pregnancy: Maternal Major Histocompatibility Complex Class I Educates Uterine Natural Killer Cells.Int J Mol Sci. 2024 Aug 15;25(16):8869. doi: 10.3390/ijms25168869. Int J Mol Sci. 2024. PMID: 39201555 Free PMC article. Review.
Cited by
-
Maternal natural killer cells at the intersection between reproduction and mucosal immunity.Mucosal Immunol. 2021 Sep;14(5):991-1005. doi: 10.1038/s41385-020-00374-3. Epub 2021 Apr 26. Mucosal Immunol. 2021. PMID: 33903735 Free PMC article. Review.
-
Deciphering decidual deficiencies in recurrent spontaneous abortion and the therapeutic potential of mesenchymal stem cells at single-cell resolution.Stem Cell Res Ther. 2024 Jul 29;15(1):228. doi: 10.1186/s13287-024-03854-6. Stem Cell Res Ther. 2024. PMID: 39075579 Free PMC article.
-
Human Listeriosis.Clin Microbiol Rev. 2023 Mar 23;36(1):e0006019. doi: 10.1128/cmr.00060-19. Epub 2022 Dec 8. Clin Microbiol Rev. 2023. PMID: 36475874 Free PMC article. Review.
-
The effects of progesterone on immune cellular function at the maternal-fetal interface and in maternal circulation.J Steroid Biochem Mol Biol. 2023 May;229:106254. doi: 10.1016/j.jsbmb.2023.106254. Epub 2023 Jan 18. J Steroid Biochem Mol Biol. 2023. PMID: 36681283 Free PMC article. Review.
-
Natural killer cell profiles in recurrent pregnancy loss: Increased expression and positive associations with TACTILE and LILRB1.Am J Reprod Immunol. 2022 Nov;88(5):e13612. doi: 10.1111/aji.13612. Epub 2022 Sep 6. Am J Reprod Immunol. 2022. PMID: 36004818 Free PMC article.
References
-
- Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Sym Soc Exp Biol. 1953;7:320–338.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

