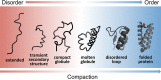
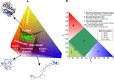
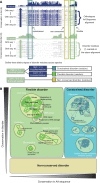
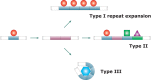
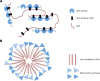
Classification of intrinsically disordered regions and proteins
- PMID: 24773235
- PMCID: PMC4095912
- DOI: 10.1021/cr400525m
Classification of intrinsically disordered regions and proteins
Figures
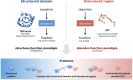
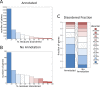
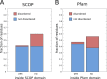





Similar articles
-
Physicochemical properties of cells and their effects on intrinsically disordered proteins (IDPs).Chem Rev. 2014 Jul 9;114(13):6661-714. doi: 10.1021/cr400695p. Epub 2014 Jun 5. Chem Rev. 2014. PMID: 24901537 Free PMC article. Review. No abstract available.
-
Functional roles of transiently and intrinsically disordered regions within proteins.FEBS J. 2015 Apr;282(7):1182-9. doi: 10.1111/febs.13202. Epub 2015 Jan 29. FEBS J. 2015. PMID: 25631540 Review.
-
Conditionally and transiently disordered proteins: awakening cryptic disorder to regulate protein function.Chem Rev. 2014 Jul 9;114(13):6779-805. doi: 10.1021/cr400459c. Epub 2014 Feb 6. Chem Rev. 2014. PMID: 24502763 Free PMC article. Review. No abstract available.
-
Sequence and Structure Properties Uncover the Natural Classification of Protein Complexes Formed by Intrinsically Disordered Proteins via Mutual Synergistic Folding.Int J Mol Sci. 2019 Nov 1;20(21):5460. doi: 10.3390/ijms20215460. Int J Mol Sci. 2019. PMID: 31683980 Free PMC article.
-
A comprehensive review and comparison of existing computational methods for intrinsically disordered protein and region prediction.Brief Bioinform. 2019 Jan 18;20(1):330-346. doi: 10.1093/bib/bbx126. Brief Bioinform. 2019. PMID: 30657889 Review.
Cited by
-
Analysis of Protein Disorder Predictions in the Light of a Protein Structural Alphabet.Biomolecules. 2020 Jul 20;10(7):1080. doi: 10.3390/biom10071080. Biomolecules. 2020. PMID: 32698546 Free PMC article.
-
SENSE-PPI reconstructs interactomes within, across, and between species at the genome scale.iScience. 2024 Jun 25;27(7):110371. doi: 10.1016/j.isci.2024.110371. eCollection 2024 Jul 19. iScience. 2024. PMID: 39055916 Free PMC article.
-
Nuclear bodies: the emerging biophysics of nucleoplasmic phases.Curr Opin Cell Biol. 2015 Jun;34:23-30. doi: 10.1016/j.ceb.2015.04.003. Epub 2015 May 15. Curr Opin Cell Biol. 2015. PMID: 25942753 Free PMC article. Review.
-
Protein conformation and biomolecular condensates.Curr Res Struct Biol. 2022 Sep 14;4:285-307. doi: 10.1016/j.crstbi.2022.09.004. eCollection 2022. Curr Res Struct Biol. 2022. PMID: 36164646 Free PMC article. Review.
-
The HSV-1 Transcription Factor ICP4 Confers Liquid-Like Properties to Viral Replication Compartments.Int J Mol Sci. 2021 Apr 24;22(9):4447. doi: 10.3390/ijms22094447. Int J Mol Sci. 2021. PMID: 33923223 Free PMC article.
References
-
- Flicek P.; Ahmed I.; Amode M. R.; Barrell D.; Beal K.; Brent S.; Carvalho-Silva D.; Clapham P.; Coates G.; Fairley S.; Fitzgerald S.; Gil L.; Garcia-Giron C.; Gordon L.; Hourlier T.; Hunt S.; Juettemann T.; Kahari A. K.; Keenan S.; Komorowska M.; Kulesha E.; Longden I.; Maurel T.; McLaren W. M.; Muffato M.; Nag R.; Overduin B.; Pignatelli M.; Pritchard B.; Pritchard E.; Riat H. S.; Ritchie G. R.; Ruffier M.; Schuster M.; Sheppard D.; Sobral D.; Taylor K.; Thormann A.; Trevanion S.; White S.; Wilder S. P.; Aken B. L.; Birney E.; Cunningham F.; Dunham I.; Harrow J.; Herrero J.; Hubbard T. J.; Johnson N.; Kinsella R.; Parker A.; Spudich G.; Yates A.; Zadissa A.; Searle S. M. Nucleic Acids Res. 2013, 41, D48. - PMC - PubMed
-
- Kolodny R.; Pereyaslavets L.; Samson A. O.; Levitt M. Annu. Rev. Biophys. 2013, 42, 559. - PubMed
-
- Raes J.; Harrington E. D.; Singh A. H.; Bork P. Curr. Opin. Struct. Biol. 2007, 17, 362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

