Molecular recognition of CCR5 by an HIV-1 gp120 V3 loop
- PMID: 24763408
- PMCID: PMC3999033
- DOI: 10.1371/journal.pone.0095767
Molecular recognition of CCR5 by an HIV-1 gp120 V3 loop
Abstract
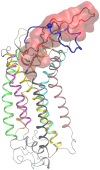
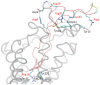
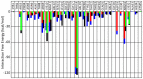
The binding of protein HIV-1 gp120 to coreceptors CCR5 or CXCR4 is a key step of the HIV-1 entry to the host cell, and is predominantly mediated through the V3 loop fragment of HIV-1 gp120. In the present work, we delineate the molecular recognition of chemokine receptor CCR5 by a dual tropic HIV-1 gp120 V3 loop, using a comprehensive set of computational tools predominantly based on molecular dynamics simulations and free energy calculations. We report, what is to our knowledge, the first complete HIV-1 gp120 V3 loop : CCR5 complex structure, which includes the whole V3 loop and the N-terminus of CCR5, and exhibits exceptional agreement with previous experimental findings. The computationally derived structure sheds light into the functional role of HIV-1 gp120 V3 loop and CCR5 residues associated with the HIV-1 coreceptor activity, and provides insights into the HIV-1 coreceptor selectivity and the blocking mechanism of HIV-1 gp120 by maraviroc. By comparing the binding of the specific dual tropic HIV-1 gp120 V3 loop with CCR5 and CXCR4, we observe that the HIV-1 gp120 V3 loop residues 13-21, which include the tip, share nearly identical structural and energetic properties in complex with both coreceptors. This result paves the way for the design of dual CCR5/CXCR4 targeted peptides as novel potential anti-AIDS therapeutics.
Conflict of interest statement
Figures
Similar articles
-
Molecular recognition of CXCR4 by a dual tropic HIV-1 gp120 V3 loop.Biophys J. 2013 Sep 17;105(6):1502-14. doi: 10.1016/j.bpj.2013.07.049. Biophys J. 2013. PMID: 24048002 Free PMC article.
-
Structure and dynamics of the gp120 V3 loop that confers noncompetitive resistance in R5 HIV-1(JR-FL) to maraviroc.PLoS One. 2013 Jun 28;8(6):e65115. doi: 10.1371/journal.pone.0065115. Print 2013. PLoS One. 2013. PMID: 23840315 Free PMC article.
-
Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex.Science. 2013 Sep 20;341(6152):1387-90. doi: 10.1126/science.1241475. Epub 2013 Sep 12. Science. 2013. PMID: 24030490 Free PMC article.
-
Effect of HIV-1 subtype and tropism on treatment with chemokine coreceptor entry inhibitors; overview of viral entry inhibition.Crit Rev Microbiol. 2015;41(4):473-87. doi: 10.3109/1040841X.2013.867829. Epub 2014 Mar 17. Crit Rev Microbiol. 2015. PMID: 24635642 Review.
-
HIV-1 gp120 V3 loop for structure-based drug design.Curr Protein Pept Sci. 2005 Oct;6(5):413-22. doi: 10.2174/138920305774329359. Curr Protein Pept Sci. 2005. PMID: 16248793 Review.
Cited by
-
Modeling of CCR5 Recognition by HIV-1 gp120: How the Viral Protein Exploits the Conformational Plasticity of the Coreceptor.Viruses. 2021 Jul 18;13(7):1395. doi: 10.3390/v13071395. Viruses. 2021. PMID: 34372601 Free PMC article.
-
Structure-Based Design and Development of Chemical Probes Targeting Putative MOR-CCR5 Heterodimers to Inhibit Opioid Exacerbated HIV-1 Infectivity.J Med Chem. 2021 Jun 10;64(11):7702-7723. doi: 10.1021/acs.jmedchem.1c00408. Epub 2021 May 23. J Med Chem. 2021. PMID: 34027668 Free PMC article.
-
Isoflavones as Ah Receptor Agonists in Colon-Derived Cell Lines: Structure-Activity Relationships.Chem Res Toxicol. 2019 Nov 18;32(11):2353-2364. doi: 10.1021/acs.chemrestox.9b00352. Epub 2019 Oct 29. Chem Res Toxicol. 2019. PMID: 31621310 Free PMC article.
-
In Vitro and In Silico Antiviral Activity of Di-Halogenated Compounds Derived from L-Tyrosine against Human Immunodeficiency Virus 1 (HIV-1).Curr Issues Mol Biol. 2023 Oct 9;45(10):8173-8200. doi: 10.3390/cimb45100516. Curr Issues Mol Biol. 2023. PMID: 37886959 Free PMC article.
-
Insights into the mechanism of C5aR inhibition by PMX53 via implicit solvent molecular dynamics simulations and docking.BMC Biophys. 2014 Aug 12;7:5. doi: 10.1186/2046-1682-7-5. eCollection 2014. BMC Biophys. 2014. PMID: 25170421 Free PMC article.
References
-
- Dittmar MT, McKnight A, Simmons G, Clapham PR, Weiss RA, et al. (1997) HIV-1 tropism and co-receptor use. Nature 385: 495–496. - PubMed
-
- Weiss RA, Clapham PR (1996) Hot fusion of HIV. Nature 381: 647–648. - PubMed
-
- Chan DC, Kim PS (1998) HIV entry and its inhibition. Cell 93: 681–684. - PubMed
-
- Chan DC, Fass D, Berger JM, Kim PS (1997) Core structure of gp41 from the HIV envelope glycoprotein. Cell 89: 263–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

