Rotavirus vaccines: current status and future considerations
- PMID: 24755452
- PMCID: PMC4185955
- DOI: 10.4161/hv.28857
Rotavirus vaccines: current status and future considerations
Abstract
Rotavirus is the leading cause of severe diarrhea among children<5 years worldwide. Currently licensed rotavirus vaccines have been efficacious and effective, with many countries reporting substantial declines in diarrheal and rotavirus-specific morbidity and mortality. However, the full public health impact of these vaccines has not been realized. Most countries, including those with the highest disease burden, have not yet introduced rotavirus vaccines into their national immunization programs. Research activities that may help inform vaccine introduction decisions include (1) establishing effectiveness, impact, and safety for rotavirus vaccines in low-income settings; (2) identifying potential strategies to improve performance of oral rotavirus vaccines in developing countries, such as zinc supplementation; and (3) pursuing alternate approaches to oral vaccines, such as parenteral immunization. Policy- and program-level barriers, such as financial implications of new vaccine introductions, should be addressed to ensure that countries are able to make informed decisions regarding rotavirus vaccine introduction.
Keywords: diarrhea; gastroenteritis; rotavirus; rotavirus vaccines; vaccine impact.
Figures
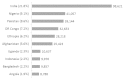
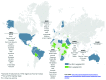
Similar articles
-
Rotavirus vaccines: successes and challenges.J Infect. 2014 Jan;68 Suppl 1:S9-18. doi: 10.1016/j.jinf.2013.09.010. Epub 2013 Oct 22. J Infect. 2014. PMID: 24156947 Review.
-
Experiences with rotavirus vaccines: can we improve rotavirus vaccine impact in developing countries?Hum Vaccin Immunother. 2019;15(6):1215-1227. doi: 10.1080/21645515.2018.1553593. Epub 2019 Feb 8. Hum Vaccin Immunother. 2019. PMID: 30735087 Free PMC article. Review.
-
Challenges to Developing a Rotavirus Vaccine.Viral Immunol. 2018 Mar;31(2):104-108. doi: 10.1089/vim.2017.0121. Epub 2017 Dec 21. Viral Immunol. 2018. PMID: 29265955 Review.
-
Remaining issues and challenges for rotavirus vaccine in preventing global childhood diarrheal morbidity and mortality.Expert Rev Vaccines. 2012 Feb;11(2):211-20. doi: 10.1586/erv.11.184. Expert Rev Vaccines. 2012. PMID: 22309669 Review.
-
Performance of rotavirus vaccines in developed and developing countries.Hum Vaccin. 2010 Jul;6(7):532-42. doi: 10.4161/hv.6.7.11278. Hum Vaccin. 2010. PMID: 20622508 Free PMC article. Review.
Cited by
-
Changes in vaccine coverage and incidence of acute gastroenteritis and severe rotavirus gastroenteritis in children <5 years in Shibata City, Niigata Prefecture, Japan.Hum Vaccin Immunother. 2024 Dec 31;20(1):2322202. doi: 10.1080/21645515.2024.2322202. Epub 2024 Mar 13. Hum Vaccin Immunother. 2024. PMID: 38478958 Free PMC article.
-
Association between Immunogenicity of a Monovalent Parenteral P2-VP8 Subunit Rotavirus Vaccine and Fecal Shedding of Rotavirus following Rotarix Challenge during a Randomized, Double-Blind, Placebo-Controlled Trial.Viruses. 2023 Aug 25;15(9):1809. doi: 10.3390/v15091809. Viruses. 2023. PMID: 37766217 Free PMC article. Clinical Trial.
-
Post-marketing safety surveillance of the rotavirus vaccine in India.Vaccine X. 2023 Aug 1;15:100362. doi: 10.1016/j.jvacx.2023.100362. eCollection 2023 Dec. Vaccine X. 2023. PMID: 37593522 Free PMC article.
-
Quercetin, a flavonoid, combats rotavirus infection by deactivating rotavirus-induced pro-survival NF-κB pathway.Front Microbiol. 2022 Aug 2;13:951716. doi: 10.3389/fmicb.2022.951716. eCollection 2022. Front Microbiol. 2022. PMID: 35983320 Free PMC article.
-
Electron-Beam Inactivation of Human Rotavirus (HRV) for the Production of Neutralizing Egg Yolk Antibodies.Front Immunol. 2022 Mar 14;13:840077. doi: 10.3389/fimmu.2022.840077. eCollection 2022. Front Immunol. 2022. PMID: 35359996 Free PMC article.
References
-
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al. Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. - DOI - PubMed
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. doi: 10.1016/S1473-3099(11)70253-5. - DOI - PubMed
-
- World Health Organization. Global rotavirus information and surveillance bulletin.2013; 7:1-11.
-
- World Health Organization/United Nations Children's Fund. Ending preventable child deaths from pneumonia and diarrhoea by 2025: the integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD). France: WHO Press; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
