Sumoylation of the astroglial glutamate transporter EAAT2 governs its intracellular compartmentalization
- PMID: 24753081
- PMCID: PMC4061158
- DOI: 10.1002/glia.22677
Sumoylation of the astroglial glutamate transporter EAAT2 governs its intracellular compartmentalization
Abstract
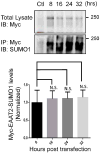
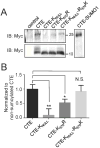
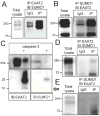
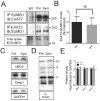
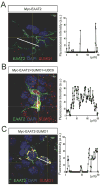
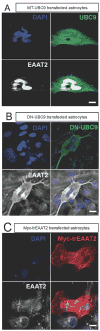
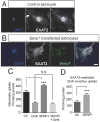
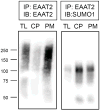
EAAT2 is a predominantly astroglial glutamate transporter responsible for the majority of synaptic glutamate clearance in the mammalian central nervous system (CNS). Its dysfunction has been linked with many neurological disorders, including amyotrophic lateral sclerosis (ALS). Decreases in EAAT2 expression and function have been implicated in causing motor neuron excitotoxic death in ALS. Nevertheless, increasing EAAT2 expression does not significantly improve ALS phenotype in mouse models or in clinical trials. In the SOD1-G93A mouse model of inherited ALS, the cytosolic carboxy-terminal domain is cleaved from EAAT2, conjugated to SUMO1, and accumulated in astrocytes where it triggers astrocyte-mediated neurotoxic effects as disease progresses. However, it is not known whether this fragment is sumoylated after cleavage or if full-length EAAT2 is already sumoylated prior to cleavage as part of physiological regulation. In this study, we show that a fraction of full-length EAAT2 is constitutively sumoylated in primary cultures of astrocytes in vitro and in the CNS in vivo. Furthermore, the extent of sumoylation of EAAT2 does not change during the course of ALS in the SOD1-G93A mouse and is not affected by the expression of ALS-causative mutant SOD1 proteins in astrocytes in vitro, indicating that EAAT2 sumoylation is not driven by pathogenic mechanisms. Most interestingly, sumoylated EAAT2 localizes to intracellular compartments, whereas non-sumoylated EAAT2 resides on the plasma membrane. In agreement, promoting desumoylation in primary astrocytes causes increased EAAT2-mediated glutamate uptake. These findings could have implications for optimizing therapeutic approaches aimed at increasing EAAT2 activity in the dysfunctional or diseased CNS.
Keywords: GLT-1; amyotrophic lateral sclerosis; excitotoxicity; protein trafficking.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
The Authors declare that they have no conflict of interest
Figures
Similar articles
-
Mutation of the caspase-3 cleavage site in the astroglial glutamate transporter EAAT2 delays disease progression and extends lifespan in the SOD1-G93A mouse model of ALS.Exp Neurol. 2017 Jun;292:145-153. doi: 10.1016/j.expneurol.2017.03.014. Epub 2017 Mar 22. Exp Neurol. 2017. PMID: 28342750 Free PMC article.
-
Motor neuron impairment mediated by a sumoylated fragment of the glial glutamate transporter EAAT2.Glia. 2011 Nov;59(11):1719-31. doi: 10.1002/glia.21218. Epub 2011 Jul 18. Glia. 2011. PMID: 21769946 Free PMC article.
-
A caspase-3-cleaved fragment of the glial glutamate transporter EAAT2 is sumoylated and targeted to promyelocytic leukemia nuclear bodies in mutant SOD1-linked amyotrophic lateral sclerosis.J Biol Chem. 2007 Nov 2;282(44):32480-90. doi: 10.1074/jbc.M704314200. Epub 2007 Sep 6. J Biol Chem. 2007. PMID: 17823119
-
Sumoylation of critical proteins in amyotrophic lateral sclerosis: emerging pathways of pathogenesis.Neuromolecular Med. 2013 Dec;15(4):760-70. doi: 10.1007/s12017-013-8262-x. Epub 2013 Sep 24. Neuromolecular Med. 2013. PMID: 24062161 Free PMC article. Review.
-
EAAT2 and the Molecular Signature of Amyotrophic Lateral Sclerosis.Adv Neurobiol. 2017;16:117-136. doi: 10.1007/978-3-319-55769-4_6. Adv Neurobiol. 2017. PMID: 28828608 Free PMC article. Review.
Cited by
-
Transcriptomics and Metabolomics in Amyotrophic Lateral Sclerosis.Adv Exp Med Biol. 2020;1195:205-212. doi: 10.1007/978-3-030-32633-3_29. Adv Exp Med Biol. 2020. PMID: 32468479 Review.
-
Rapid recycling of glutamate transporters on the astroglial surface.Elife. 2021 Apr 16;10:e64714. doi: 10.7554/eLife.64714. Elife. 2021. PMID: 33860761 Free PMC article.
-
Evidence of adaptation of maternofetal transport of glutamine relative to placental size in normal mice, and in those with fetal growth restriction.J Physiol. 2019 Oct;597(19):4975-4990. doi: 10.1113/JP278226. Epub 2019 Aug 27. J Physiol. 2019. PMID: 31400764 Free PMC article.
-
Chronic Unexpected Mild Stress Destroys Synaptic Plasticity of Neurons through a Glutamate Transporter, GLT-1, of Astrocytes in the Ischemic Stroke Rat.Neural Plast. 2019 Mar 25;2019:1615925. doi: 10.1155/2019/1615925. eCollection 2019. Neural Plast. 2019. PMID: 31019528 Free PMC article.
-
Glutamate Transport: A New Bench to Bedside Mechanism for Treating Drug Abuse.Int J Neuropsychopharmacol. 2017 Oct 1;20(10):797-812. doi: 10.1093/ijnp/pyx050. Int J Neuropsychopharmacol. 2017. PMID: 28605494 Free PMC article. Review.
References
-
- Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH, Jr, Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006;281:14076–84. - PubMed
-
- Bristol LA, Rothstein JD. Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Ann Neurol. 1996;39:676–9. - PubMed
-
- Butchbach ME, Tian G, Guo H, Lin CL. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function. J Biol Chem. 2004;279:34388–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

