Deregulation of the actin cytoskeleton and macropinocytosis in response to phorbol ester by the mutant protein kinase C gamma that causes spinocerebellar ataxia type 14
- PMID: 24744737
- PMCID: PMC3978357
- DOI: 10.3389/fphys.2014.00126
Deregulation of the actin cytoskeleton and macropinocytosis in response to phorbol ester by the mutant protein kinase C gamma that causes spinocerebellar ataxia type 14
Abstract
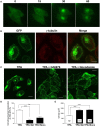
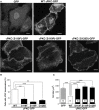
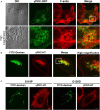
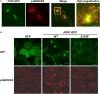
Several missense mutations in the protein kinase Cγ (γPKC) gene have been found to cause spinocerebellar ataxia type 14 (SCA14), an autosomal dominant neurodegenerative disease. γPKC is a neuron-specific member of the classical PKCs and is activated and translocated to subcellular regions as a result of various stimuli, including diacylglycerol synthesis, increased intracellular Ca(2+) and phorbol esters. We investigated whether SCA14 mutations affect the γPKC-related functions by stimulating HeLa cells with TPA (12-O-tetradecanoylpholbol 13-acetate), a type of phorbol ester. Wild-type (WT) γPKC-GFP was translocated to the plasma membrane within 10 min of TPA stimulation, followed by its perinuclear translocation and cell shrinkage, in a PKC kinase activity- and microtubule-dependent manner. On the other hand, although SCA14 mutant γPKC-GFP exhibited a similar translocation to the plasma membrane, the subsequent perinuclear translocation and cell shrinkage were significantly impaired in response to TPA. Translocated WT γPKC colocalized with F-actin and formed large vesicular structures in the perinuclear region. The uptake of FITC-dextran, a marker of macropinocytosis, was promoted by TPA stimulation in cells expressing WT γPKC, and FITC-dextran was surrounded by γPKC-positive vesicles. Moreover, TPA induced the phosphorylation of MARCKS, which is a membrane-substrate of PKC, resulting in the translocation of phosphorylated MARCKS to the perinuclear region, suggesting that TPA induces macropinocytosis via γPKC activation. However, TPA failed to activate macropinocytosis and trigger the translocation of phosphorylated MARCKS in cells expressing the SCA14 mutant γPKC. These findings suggest that γPKC is involved in the regulation of the actin cytoskeleton and macropinocytosis in HeLa cells, while SCA14 mutant γPKC fails to regulate these processes due to its reduced kinase activity at the plasma membrane. This property might be involved in pathogenesis of SCA14.
Keywords: MARCKS; actin cytoskeleton; macropinocytosis; spinocerebellar ataxia type 14; translocation; γPKC.
Figures
Similar articles
-
Mutant protein kinase Cgamma found in spinocerebellar ataxia type 14 is susceptible to aggregation and causes cell death.J Biol Chem. 2005 Aug 12;280(32):29096-106. doi: 10.1074/jbc.M501716200. Epub 2005 Jun 17. J Biol Chem. 2005. PMID: 15964845
-
Mutant protein kinase C gamma that causes spinocerebellar ataxia type 14 (SCA14) is selectively degraded by autophagy.Genes Cells. 2010 May;15(5):425-38. doi: 10.1111/j.1365-2443.2010.01395.x. Epub 2010 Apr 11. Genes Cells. 2010. PMID: 20398063
-
Mutant gammaPKC found in spinocerebellar ataxia type 14 induces aggregate-independent maldevelopment of dendrites in primary cultured Purkinje cells.Neurobiol Dis. 2009 Feb;33(2):260-73. doi: 10.1016/j.nbd.2008.10.013. Epub 2008 Nov 8. Neurobiol Dis. 2009. PMID: 19041943
-
Elucidation of the molecular mechanism and exploration of novel therapeutics for spinocerebellar ataxia caused by mutant protein kinase Cγ.J Pharmacol Sci. 2011;116(3):239-47. doi: 10.1254/jphs.11r04cp. Epub 2011 Jun 10. J Pharmacol Sci. 2011. PMID: 21666345 Review.
-
Two Sides of the Same Coin: Protein Kinase C γ in Cancer and Neurodegeneration.Front Cell Dev Biol. 2022 Jun 21;10:929510. doi: 10.3389/fcell.2022.929510. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35800893 Free PMC article. Review.
Cited by
-
Widespread alternative splicing dysregulation occurs presymptomatically in CAG expansion spinocerebellar ataxias.Brain. 2024 Feb 1;147(2):486-504. doi: 10.1093/brain/awad329. Brain. 2024. PMID: 37776516 Free PMC article.
-
Delta Opioid Receptor Activation with Delta Opioid Peptide [d-Ala2, d-Leu5] Enkephalin Contributes to Synaptic Improvement in Rat Hippocampus against Global Ischemia.Cell Transplant. 2021 Jan-Dec;30:9636897211041585. doi: 10.1177/09636897211041585. Cell Transplant. 2021. PMID: 34470528 Free PMC article.
-
Activated Protein Kinase C (PKC) Is Persistently Trafficked with Epidermal Growth Factor (EGF) Receptor.Biomolecules. 2020 Sep 7;10(9):1288. doi: 10.3390/biom10091288. Biomolecules. 2020. PMID: 32906765 Free PMC article.
-
Low Electric Treatment activates Rho GTPase via Heat Shock Protein 90 and Protein Kinase C for Intracellular Delivery of siRNA.Sci Rep. 2019 Mar 11;9(1):4114. doi: 10.1038/s41598-019-40904-z. Sci Rep. 2019. PMID: 30858501 Free PMC article.
-
Roles of Post-translational Modifications in Spinocerebellar Ataxias.Front Cell Neurosci. 2018 Sep 19;12:290. doi: 10.3389/fncel.2018.00290. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30283301 Free PMC article. Review.
References
-
- Aballay A., Stahl P. D., Mayorga L. S. (1999). Phorbol ester promotes endocytosis by activating a factor involved in endosome fusion. J. Cell Sci. 112(Pt 15), 2549–2557 - PubMed
-
- Alvi F., Idkowiak-Baldys J., Baldys A., Raymond J. R., Hannun Y. A. (2007). Regulation of membrane trafficking and endocytosis by protein kinase C: emerging role of the pericentrion, a novel protein kinase C-dependent subset of recycling endosomes. Cell. Mol. Life Sci. 64, 263–270 10.1007/s00018-006-6363-5 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

