Pharmacological ceramide reduction alleviates alcohol-induced steatosis and hepatomegaly in adiponectin knockout mice
- PMID: 24742988
- PMCID: PMC4042116
- DOI: 10.1152/ajpgi.00395.2013
Pharmacological ceramide reduction alleviates alcohol-induced steatosis and hepatomegaly in adiponectin knockout mice
Abstract
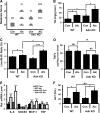
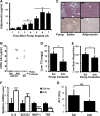
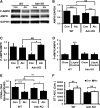
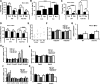
Hepatosteatosis, the ectopic accumulation of lipid in the liver, is one of the earliest clinical signs of alcoholic liver disease (ALD). Alcohol-dependent deregulation of liver ceramide levels as well as inhibition of AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor α (PPAR-α) activity are thought to contribute to hepatosteatosis development. Adiponectin can regulate lipid handling in the liver and has been shown to reduce ceramide levels and activate AMPK and PPAR-α. However, the mechanisms by which adiponectin prevents alcoholic hepatosteatosis remain incompletely characterized. To address this question, we assessed ALD progression in wild-type (WT) and adiponectin knockout (KO) mice fed an ethanol-containing liquid diet or isocaloric control diet. Adiponectin KO mice relative to WT had increased alcohol-induced hepatosteatosis and hepatomegaly, similar modest increases in serum alanine aminotransferase, and reduced liver TNF. Restoring circulating adiponectin levels using recombinant adiponectin ameliorated alcohol-induced hepatosteatosis and hepatomegaly in adiponectin KO mice. Alcohol-fed WT and adiponectin KO animals had equivalent reductions in AMPK protein and PPAR-α DNA binding activity compared with control-fed animals. No difference in P-AMPK/AMPK ratio was detected, suggesting that alcohol-dependent deregulation of AMPK and PPAR-α in the absence of adiponectin are not primary causes of the observed increase in hepatosteatosis in these animals. By contrast, alcohol treatment increased liver ceramide levels in adiponectin KO but not WT mice. Importantly, pharmacological inhibition of de novo ceramide synthesis in adiponectin KO mice abrogated alcohol-mediated increases in liver ceramides, steatosis, and hepatomegaly. These data suggest that adiponectin reduces alcohol-induced steatosis and hepatomegaly through regulation of liver ceramides, but its absence does not exacerbate alcohol-induced liver damage.
Keywords: adiponectin; alcohol; ceramide; hepatosteatosis.
Copyright © 2014 the American Physiological Society.
Figures
Similar articles
-
Ceramide synthase 6 (CerS6) is upregulated in alcohol-associated liver disease and exhibits sex-based differences in the regulation of energy homeostasis and lipid droplet accumulation.Mol Metab. 2023 Dec;78:101804. doi: 10.1016/j.molmet.2023.101804. Epub 2023 Sep 14. Mol Metab. 2023. PMID: 37714377 Free PMC article.
-
Resveratrol alleviates alcoholic fatty liver in mice.Am J Physiol Gastrointest Liver Physiol. 2008 Oct;295(4):G833-42. doi: 10.1152/ajpgi.90358.2008. Epub 2008 Aug 28. Am J Physiol Gastrointest Liver Physiol. 2008. PMID: 18755807 Free PMC article.
-
Methyl ferulic acid attenuates ethanol-induced hepatic steatosis by regulating AMPK and FoxO1 Pathways in Rats and L-02 cells.Chem Biol Interact. 2018 Aug 1;291:180-189. doi: 10.1016/j.cbi.2018.06.028. Epub 2018 Jun 27. Chem Biol Interact. 2018. PMID: 29940154
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
-
Alcohol-induced steatosis in liver cells.World J Gastroenterol. 2007 Oct 7;13(37):4974-8. doi: 10.3748/wjg.v13.i37.4974. World J Gastroenterol. 2007. PMID: 17854140 Free PMC article. Review.
Cited by
-
Signal Transduction Mechanisms of Alcoholic Fatty Liver Disease: Emer ging Role of Lipin-1.Curr Mol Pharmacol. 2017;10(3):226-236. doi: 10.2174/1874467208666150817112109. Curr Mol Pharmacol. 2017. PMID: 26278388 Free PMC article. Review.
-
Multi-technique comparison of atherogenic and MCD NASH models highlights changes in sphingolipid metabolism.Sci Rep. 2019 Nov 14;9(1):16810. doi: 10.1038/s41598-019-53346-4. Sci Rep. 2019. PMID: 31728041 Free PMC article.
-
Contribution of Hepatic Steatosis-Intensified Extracellular Vesicle Release to Aggravated Inflammatory Endothelial Injury in Liver-Specific Asah1 Gene Knockout Mice.Am J Pathol. 2023 Apr;193(4):493-508. doi: 10.1016/j.ajpath.2022.12.007. Epub 2023 Jan 10. Am J Pathol. 2023. PMID: 36638912 Free PMC article.
-
Ceramides: Nutrient Signals that Drive Hepatosteatosis.J Lipid Atheroscler. 2020 Jan;9(1):50-65. doi: 10.12997/jla.2020.9.1.50. Epub 2019 Nov 13. J Lipid Atheroscler. 2020. PMID: 32821721 Free PMC article. Review.
-
AdipoAtlas: A reference lipidome for human white adipose tissue.Cell Rep Med. 2021 Sep 22;2(10):100407. doi: 10.1016/j.xcrm.2021.100407. eCollection 2021 Oct 19. Cell Rep Med. 2021. PMID: 34755127 Free PMC article.
References
-
- Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology 20: 453–460, 1994 - PubMed
-
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 108: 218–224, 1995 - PubMed
-
- Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol 8: 491–501, 2011 - PubMed
-
- An L, Wang X, Cederbaum AI. Cytokines in alcoholic liver disease. Arch Toxicol 86: 1337–1348, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

