Anti-CD3 antibody treatment induces hypoglycemia and super tolerance to glucose challenge in mice through enhancing glucose consumption by activated lymphocytes
- PMID: 24741590
- PMCID: PMC3987876
- DOI: 10.1155/2014/326708
Anti-CD3 antibody treatment induces hypoglycemia and super tolerance to glucose challenge in mice through enhancing glucose consumption by activated lymphocytes
Abstract
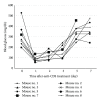
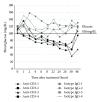
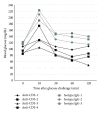
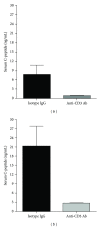
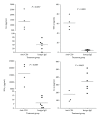
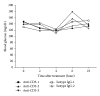
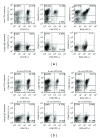
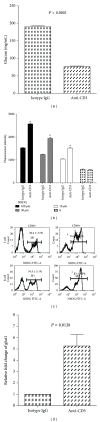
Anti-CD3 antibody has been employed for various immune-mediated disorders. However, whether anti-CD3 administration leads to rapid metabolic alternation has not been well investigated. In the current study, we studied how anti-CD3 treatment affected blood glucose levels in mice. We found that anti-CD3 treatment induced immediate reduction of blood glucose after administration. Furthermore, a single dose of anti-CD3 treatment corrected hyperglycemia in all nonobese diabetic mice with recently diagnosed diabetes. This glucose-lowering effect was not attributable to major T cell produced cytokines. Of interest, when tested in a normal strain of mice (C57BL/6), the serum levels of C-peptide in anti-CD3 treated animals were significantly lower than control mice. Paradoxically, anti-CD3 treated animals were highly tolerant to exogenous glucose challenge. Additionally, we found that anti-CD3 treatment significantly induced activation of T and B cells in vitro and in vivo. Further studies demonstrated that anti-CD3 treatment lowered the glucose levels in T cell culture media and increased the intracellular transportation of 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2 deoxyglucose (2-NBDG) particularly in activated T and B cells. In addition, injection of anti-CD3 antibodies induced enhanced levels of Glut1 expression in spleen cells. This study suggests that anti-CD3 therapy-induced hypoglycemia likely results from increased glucose transportation and consumption by the activated lymphocytes.
Figures

Similar articles
-
Induction of active tolerance and involvement of CD1d-restricted natural killer T cells in anti-CD3 F(ab')2 treatment-reversed new-onset diabetes in nonobese diabetic mice.Am J Pathol. 2008 Apr;172(4):972-9. doi: 10.2353/ajpath.2008.070159. Epub 2008 Mar 18. Am J Pathol. 2008. PMID: 18349126 Free PMC article.
-
Perturbed homeostasis of peripheral T cells elicits decreased susceptibility to anti-CD3-induced apoptosis in prediabetic nonobese diabetic mice.J Immunol. 2004 Oct 1;173(7):4407-16. doi: 10.4049/jimmunol.173.7.4407. J Immunol. 2004. PMID: 15383571
-
Addition of rapamycin to anti-CD3 antibody improves long-term glycaemia control in diabetic NOD mice.PLoS One. 2013 Jun 24;8(6):e67189. doi: 10.1371/journal.pone.0067189. Print 2013. PLoS One. 2013. PMID: 23826229 Free PMC article.
-
CD3 monoclonal antibodies: a first step towards operational immune tolerance in the clinic.Rev Diabet Stud. 2012 Winter;9(4):372-81. doi: 10.1900/RDS.2012.9.372. Epub 2012 Dec 28. Rev Diabet Stud. 2012. PMID: 23804274 Free PMC article. Review.
-
CD3 antibody treatment stimulates the functional capability of regulatory T cells.Novartis Found Symp. 2003;252:279-86; discussion 286-90. Novartis Found Symp. 2003. PMID: 14609225 Review.
Cited by
-
Spatially resolved measurement of dynamic glucose uptake in live ex vivo tissues.Anal Chim Acta. 2021 Jan 2;1141:47-56. doi: 10.1016/j.aca.2020.10.027. Epub 2020 Oct 20. Anal Chim Acta. 2021. PMID: 33248661 Free PMC article.
-
Lymphocyte-Related Immunomodulatory Therapy with Siponimod (BAF-312) Improves Outcomes in Mice with Acute Intracerebral Hemorrhage.Aging Dis. 2023 Jun 1;14(3):966-991. doi: 10.14336/AD.2022.1102. Aging Dis. 2023. PMID: 37191423 Free PMC article.
-
Role of T cells in malnutrition and obesity.Front Immunol. 2014 Aug 11;5:379. doi: 10.3389/fimmu.2014.00379. eCollection 2014. Front Immunol. 2014. PMID: 25157251 Free PMC article. Review.
References
-
- Yu X-Z, Levin SD, Madrenas J, Anasetti C. Lck is required for activation-induced T cell death after TCR ligation with partial agonists. Journal of Immunology. 2004;172(3):1437–1443. - PubMed
-
- You S, Leforban B, Garcia C, Bach J-F, Bluestone JA, Chatenoud L. Adaptive TGF-β-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(15):6335–6340. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

