ADCY5 couples glucose to insulin secretion in human islets
- PMID: 24740569
- PMCID: PMC4141364
- DOI: 10.2337/db13-1607
ADCY5 couples glucose to insulin secretion in human islets
Abstract
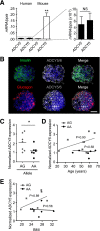
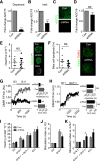
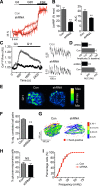
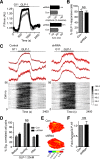
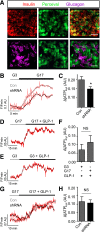
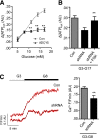
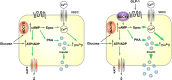
Single nucleotide polymorphisms (SNPs) within the ADCY5 gene, encoding adenylate cyclase 5, are associated with elevated fasting glucose and increased type 2 diabetes (T2D) risk. Despite this, the mechanisms underlying the effects of these polymorphic variants at the level of pancreatic β-cells remain unclear. Here, we show firstly that ADCY5 mRNA expression in islets is lowered by the possession of risk alleles at rs11708067. Next, we demonstrate that ADCY5 is indispensable for coupling glucose, but not GLP-1, to insulin secretion in human islets. Assessed by in situ imaging of recombinant probes, ADCY5 silencing impaired glucose-induced cAMP increases and blocked glucose metabolism toward ATP at concentrations of the sugar >8 mmol/L. However, calcium transient generation and functional connectivity between individual human β-cells were sharply inhibited at all glucose concentrations tested, implying additional, metabolism-independent roles for ADCY5. In contrast, calcium rises were unaffected in ADCY5-depleted islets exposed to GLP-1. Alterations in β-cell ADCY5 expression and impaired glucose signaling thus provide a likely route through which ADCY5 gene polymorphisms influence fasting glucose levels and T2D risk, while exerting more minor effects on incretin action.
© 2014 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures
Similar articles
-
A Type 2 Diabetes-Associated Functional Regulatory Variant in a Pancreatic Islet Enhancer at the ADCY5 Locus.Diabetes. 2017 Sep;66(9):2521-2530. doi: 10.2337/db17-0464. Epub 2017 Jul 6. Diabetes. 2017. PMID: 28684635 Free PMC article.
-
ADCY5 gene expression in adipose tissue is related to obesity in men and mice.PLoS One. 2015 Mar 20;10(3):e0120742. doi: 10.1371/journal.pone.0120742. eCollection 2015. PLoS One. 2015. PMID: 25793868 Free PMC article.
-
Adenylyl cyclase 8 is central to glucagon-like peptide 1 signalling and effects of chronically elevated glucose in rat and human pancreatic beta cells.Diabetologia. 2011 Feb;54(2):390-402. doi: 10.1007/s00125-010-1955-x. Epub 2010 Nov 3. Diabetologia. 2011. PMID: 21046358
-
cAMP signalling in insulin and glucagon secretion.Diabetes Obes Metab. 2017 Sep;19 Suppl 1:42-53. doi: 10.1111/dom.12993. Diabetes Obes Metab. 2017. PMID: 28466587 Review.
-
Glucagon-like peptide 1-potentiated insulin secretion and proliferation of pancreatic β-cells.J Diabetes. 2014 Sep;6(5):394-402. doi: 10.1111/1753-0407.12161. Epub 2014 May 22. J Diabetes. 2014. PMID: 24725840 Review.
Cited by
-
Metabolomics in Diabetic Retinopathy: From Potential Biomarkers to Molecular Basis of Oxidative Stress.Cells. 2022 Sep 26;11(19):3005. doi: 10.3390/cells11193005. Cells. 2022. PMID: 36230967 Free PMC article. Review.
-
Partial agonism improves the anti-hyperglycaemic efficacy of an oxyntomodulin-derived GLP-1R/GCGR co-agonist.Mol Metab. 2021 Sep;51:101242. doi: 10.1016/j.molmet.2021.101242. Epub 2021 Apr 30. Mol Metab. 2021. PMID: 33933675 Free PMC article.
-
In Vivo ZIMIR Imaging of Mouse Pancreatic Islet Cells Shows Oscillatory Insulin Secretion.Front Endocrinol (Lausanne). 2021 Mar 9;12:613964. doi: 10.3389/fendo.2021.613964. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33767668 Free PMC article.
-
Transcriptomic Profiling of Gene Expression Associated with Granulosa Cell Tumor Development in a Mouse Model.Cancers (Basel). 2022 Apr 27;14(9):2184. doi: 10.3390/cancers14092184. Cancers (Basel). 2022. PMID: 35565312 Free PMC article.
-
Risk Alleles in/near ADCY5, ADRA2A, CDKAL1, CDKN2A/B, GRB10, and TCF7L2 Elevate Plasma Glucose Levels at Birth and in Early Childhood: Results from the FAMILY Study.PLoS One. 2016 Apr 6;11(4):e0152107. doi: 10.1371/journal.pone.0152107. eCollection 2016. PLoS One. 2016. PMID: 27049325 Free PMC article.
References
-
- Scully T. Diabetes in numbers. Nature 2012;485:S2–S3 - PubMed
-
- Smith U, Gale EA. Cancer and diabetes: are we ready for prime time? Diabetologia 2010;53:1541–1544 - PubMed
-
- DeFronzo RA, Abdul-Ghani MA. Preservation of β-cell function: the key to diabetes prevention. J Clin Endocrinol Metab 2011;96:2354–2366 - PubMed
-
- Rutter GA, Parton LE. The beta-cell in type 2 diabetes and in obesity. Front Horm Res 2008;36:118–134 - PubMed
-
- McCulloch LJ, van de Bunt M, Braun M, Frayn KN, Clark A, Gloyn AL. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: implications for understanding genetic association signals at this locus. Mol Genet Metab 2011;104:648–653 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

