Dynamic localization of glucokinase and its regulatory protein in hypothalamic tanycytes
- PMID: 24739934
- PMCID: PMC3989220
- DOI: 10.1371/journal.pone.0094035
Dynamic localization of glucokinase and its regulatory protein in hypothalamic tanycytes
Expression of concern in
-
Expression of Concern: Dynamic Localization of Glucokinase and Its Regulatory Protein in Hypothalamic Tanycytes.PLoS One. 2025 Jan 30;20(1):e0318865. doi: 10.1371/journal.pone.0318865. eCollection 2025. PLoS One. 2025. PMID: 39883673 Free PMC article. No abstract available.
Abstract
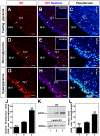
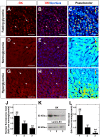
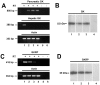
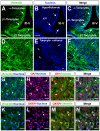
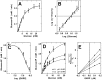
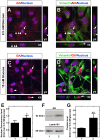
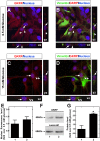
Glucokinase (GK), the hexokinase involved in glucose sensing in pancreatic β cells, is also expressed in hypothalamic tanycytes, which cover the ventricular walls of the basal hypothalamus and are implicated in an indirect control of neuronal activity by glucose. Previously, we demonstrated that GK was preferentially localized in tanycyte nuclei in euglycemic rats, which has been reported in hepatocytes and is suggestive of the presence of the GK regulatory protein, GKRP. In the present study, GK intracellular localization in hypothalamic and hepatic tissues of the same rats under several glycemic conditions was compared using confocal microscopy and Western blot analysis. In the hypothalamus, increased GK nuclear localization was observed in hyperglycemic conditions; however, it was primarily localized in the cytoplasm in hepatic tissue under the same conditions. Both GK and GKRP were next cloned from primary cultures of tanycytes. Expression of GK by Escherichia coli revealed a functional cooperative protein with a S0.5 of 10 mM. GKRP, expressed in Saccharomyces cerevisiae, inhibited GK activity in vitro with a Ki 0.2 µM. We also demonstrated increased nuclear reactivity of both GK and GKRP in response to high glucose concentrations in tanycyte cultures. These data were confirmed using Western blot analysis of nuclear extracts. Results indicate that GK undergoes short-term regulation by nuclear compartmentalization. Thus, in tanycytes, GK can act as a molecular switch to arrest cellular responses to increased glucose.
Conflict of interest statement
Figures
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Histology, Axon.2022 Nov 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2022 Nov 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 32119275 Free Books & Documents.
-
Nuclear Medicine Musculoskeletal Assessment, Protocols, and Interpretation.2022 Oct 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2022 Oct 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 35015399 Free Books & Documents.
-
Gallium Scan.2022 Dec 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2022 Dec 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 33620825 Free Books & Documents.
-
Exploring conceptual and theoretical frameworks for nurse practitioner education: a scoping review protocol.JBI Database System Rev Implement Rep. 2015 Oct;13(10):146-55. doi: 10.11124/jbisrir-2015-2150. JBI Database System Rev Implement Rep. 2015. PMID: 26571290
Cited by
-
The role of tanycytes in hypothalamic glucosensing.J Cell Mol Med. 2015 Jul;19(7):1471-82. doi: 10.1111/jcmm.12590. Epub 2015 Jun 17. J Cell Mol Med. 2015. PMID: 26081217 Free PMC article. Review.
-
GKRP-dependent modulation of feeding behavior by tanycyte-released monocarboxylates.Theranostics. 2022 Jan 3;12(4):1518-1536. doi: 10.7150/thno.66634. eCollection 2022. Theranostics. 2022. PMID: 35198055 Free PMC article.
-
Brain Glucose-Sensing Mechanism and Energy Homeostasis.Mol Neurobiol. 2019 Feb;56(2):769-796. doi: 10.1007/s12035-018-1099-4. Epub 2018 May 24. Mol Neurobiol. 2019. PMID: 29796992 Review.
-
Glial hypothalamic inhibition of GLUT2 expression alters satiety, impacting eating behavior.Glia. 2018 Mar;66(3):592-605. doi: 10.1002/glia.23267. Epub 2017 Nov 27. Glia. 2018. PMID: 29178321 Free PMC article.
-
Nutrient Sensing by Hypothalamic Tanycytes.Front Endocrinol (Lausanne). 2019 Apr 16;10:244. doi: 10.3389/fendo.2019.00244. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31040827 Free PMC article. Review.
References
-
- Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404: 661–671. - PubMed
-
- Song Z, Routh VH (2005) Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 54: 15–22. - PubMed
-
- Yang XJ, Kow LM, Funabashi T, Mobbs CV (1999) Hypothalamic glucose sensor: similarities to and differences from pancreatic beta-cell mechanisms. Diabetes 48: 1763–1772. - PubMed
-
- Chauvet N, Parmentier ML, Alonso G (1995) Transected axons of adult hypothalamo-neurohypophysial neurons regenerate along tanycytic processes. J Neurosci Res 41: 129–144. - PubMed
-
- Flament-Durand J, Brion JP (1985) Tanycytes: morphology and functions: a review. Int Rev Cytol 96: 121–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

