MiR-330-mediated regulation of SH3GL2 expression enhances malignant behaviors of glioblastoma stem cells by activating ERK and PI3K/AKT signaling pathways
- PMID: 24736727
- PMCID: PMC3988141
- DOI: 10.1371/journal.pone.0095060
MiR-330-mediated regulation of SH3GL2 expression enhances malignant behaviors of glioblastoma stem cells by activating ERK and PI3K/AKT signaling pathways
Abstract
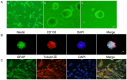
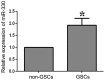
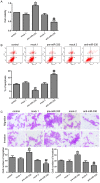
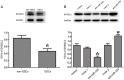
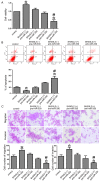
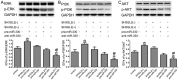
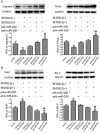
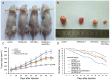
MicroRNAs are currently considered as an active and rapidly evolving area for the treatment of tumors. In this study, we elucidated the biological significance of miR-330 in glioblastoma stem cells (GSCs) as well as the possible molecular mechanisms. SH3GL2 is mainly distributed in the central nervous system and considered to be a tumor suppressor in many tumors. In the present study, we identified miR-330 as a potential regulator of SH3GL2 and we found that it was to be inversely correlated with SH3GL2 expression in GSCs which were isolated from U87 cell lines. The expression of miR-330 enhanced cellular proliferation, promoted cell migration and invasion, and dampened cell apoptosis. When the GSCs were co-transfected with the plasmid containing short hairpin RNA directed against human SH3GL2 gene and miR-330 mimic, we found that miR-330 promoted the malignant behavior of GSCs by down-regulating the expression of SH3GL2. Meanwhile, the ERK and PI3K/AKT signaling pathways were significantly activated, leading to the decreased expression of apoptotic protein and increased expression of anti-apoptotic protein. Furthermore, in orthotopic mouse xenografts, the mice given stable over-expressed SH3GL2 cells co-transfected with miR-330 knockdown plasmid had the smallest tumor sizes and longest survival. In conclusion, these results suggested that miR-330 negatively regulated the expression of SH3GL2 in GSCs, which promoted the oncogenic progression of GSCs through activating ERK and PI3K/AKT signaling pathways. The elucidation of these mechanisms will provide potential therapeutic approaches for human glioblastoma.
Conflict of interest statement
Figures
Similar articles
-
MiR-449a exerts tumor-suppressive functions in human glioblastoma by targeting Myc-associated zinc-finger protein.Mol Oncol. 2015 Mar;9(3):640-56. doi: 10.1016/j.molonc.2014.11.003. Epub 2014 Nov 20. Mol Oncol. 2015. PMID: 25487955 Free PMC article.
-
MiR-152 functions as a tumor suppressor in glioblastoma stem cells by targeting Krüppel-like factor 4.Cancer Lett. 2014 Dec 1;355(1):85-95. doi: 10.1016/j.canlet.2014.09.012. Epub 2014 Sep 10. Cancer Lett. 2014. PMID: 25218589
-
Knockdown of SOX2OT inhibits the malignant biological behaviors of glioblastoma stem cells via up-regulating the expression of miR-194-5p and miR-122.Mol Cancer. 2017 Nov 13;16(1):171. doi: 10.1186/s12943-017-0737-1. Mol Cancer. 2017. PMID: 29132362 Free PMC article.
-
Integrative analysis of cell adhesion molecules in glioblastoma identified prostaglandin F2 receptor inhibitor (PTGFRN) as an essential gene.BMC Cancer. 2022 Jun 11;22(1):642. doi: 10.1186/s12885-022-09682-2. BMC Cancer. 2022. PMID: 35690717 Free PMC article. Review.
-
Cell cultures in assessing radioresistance of glioblastomas.Zh Vopr Neirokhir Im N N Burdenko. 2022;86(5):126-132. doi: 10.17116/neiro202286051126. Zh Vopr Neirokhir Im N N Burdenko. 2022. PMID: 36252203 Review. English, Russian.
Cited by
-
Non-coding RNAs and glioma: Focus on cancer stem cells.Mol Ther Oncolytics. 2022 Sep 17;27:100-123. doi: 10.1016/j.omto.2022.09.005. eCollection 2022 Dec 15. Mol Ther Oncolytics. 2022. PMID: 36321132 Free PMC article. Review.
-
Small molecules with huge impacts: the role of miRNA-regulated PI3K pathway in human malignancies.Mol Biol Rep. 2021 Dec;48(12):8045-8059. doi: 10.1007/s11033-021-06739-6. Epub 2021 Oct 23. Mol Biol Rep. 2021. PMID: 34689281 Review.
-
MicroRNAs, Hypoxia and the Stem-Like State as Contributors to Cancer Aggressiveness.Front Genet. 2019 Feb 20;10:125. doi: 10.3389/fgene.2019.00125. eCollection 2019. Front Genet. 2019. PMID: 30842790 Free PMC article. Review.
-
A Role for the Cavin-3/Matrix Metalloproteinase-9 Signaling Axis in the Regulation of PMA-Activated Human HT1080 Fibrosarcoma Cell Neoplastic Phenotype.Cancer Growth Metastasis. 2014 Dec 8;7:43-51. doi: 10.4137/CGM.S18581. eCollection 2014. Cancer Growth Metastasis. 2014. PMID: 25520561 Free PMC article.
-
The circular RNA circFARSA sponges microRNA-330-5p in tumor cells with bladder cancer phenotype.BMC Cancer. 2022 Apr 8;22(1):373. doi: 10.1186/s12885-022-09467-7. BMC Cancer. 2022. PMID: 35395756 Free PMC article.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996. - PubMed
-
- Fukaya R, Ohta S, Yamaguchi M, Fujii H, Kawakami Y, et al. (2010) Isolation of cancer stem-like cells from a side population of a human glioblastoma cell line, SK-MG-1. Cancer Lett 291: 150–157. - PubMed
-
- Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, et al. (2009) Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell 4: 568–580. - PubMed
-
- Garzon R, Calin GA, Croce CM (2009) MicroRNAs in Cancer. Annu Rev Med 60: 167–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

