Molecular insights into NF2/Merlin tumor suppressor function
- PMID: 24726726
- PMCID: PMC4111995
- DOI: 10.1016/j.febslet.2014.04.001
Molecular insights into NF2/Merlin tumor suppressor function
Abstract
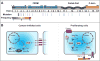
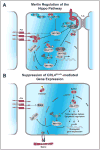
The FERM domain protein Merlin, encoded by the NF2 tumor suppressor gene, regulates cell proliferation in response to adhesive signaling. The growth inhibitory function of Merlin is induced by intercellular adhesion and inactivated by joint integrin/receptor tyrosine kinase signaling. Merlin contributes to the formation of cell junctions in polarized tissues, activates anti-mitogenic signaling at tight-junctions, and inhibits oncogenic gene expression. Thus, inactivation of Merlin causes uncontrolled mitogenic signaling and tumorigenesis. Merlin's predominant tumor suppressive functions are attributable to its control of oncogenic gene expression through regulation of Hippo signaling. Notably, Merlin translocates to the nucleus where it directly inhibits the CRL4(DCAF1) E3 ubiquitin ligase, thereby suppressing inhibition of the Lats kinases. A dichotomy in NF2 function has emerged whereby Merlin acts at the cell cortex to organize cell junctions and propagate anti-mitogenic signaling, whereas it inhibits oncogenic gene expression through the inhibition of CRL4(DCAF1) and activation of Hippo signaling. The biochemical events underlying Merlin's normal function and tumor suppressive activity will be discussed in this Review, with emphasis on recent discoveries that have greatly influenced our understanding of Merlin biology.
Keywords: CRL4 E3 ubiquitin ligase; Contact inhibition; DDB1 and Cul4-Associated Factor 1; Hippo signaling pathway; Merlin; Neurofibromatosis Type 2.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus.Cell. 2010 Feb 19;140(4):477-90. doi: 10.1016/j.cell.2010.01.029. Cell. 2010. PMID: 20178741 Free PMC article.
-
Merlin/NF2 functions upstream of the nuclear E3 ubiquitin ligase CRL4DCAF1 to suppress oncogenic gene expression.Sci Signal. 2011 Aug 23;4(188):pt6. doi: 10.1126/scisignal.2002314. Sci Signal. 2011. PMID: 21878678
-
Merlin's tumor suppression linked to inhibition of the E3 ubiquitin ligase CRL4 (DCAF1).Cell Cycle. 2010 Nov 15;9(22):4433-6. doi: 10.4161/cc.9.22.13838. Epub 2010 Nov 15. Cell Cycle. 2010. PMID: 21084862 Free PMC article.
-
Merlin: a tumour suppressor with functions at the cell cortex and in the nucleus.EMBO Rep. 2012 Mar;13(3):204-15. doi: 10.1038/embor.2012.11. EMBO Rep. 2012. PMID: 22482125 Free PMC article. Review.
-
Merlin, a multi-suppressor from cell membrane to the nucleus.FEBS Lett. 2012 May 21;586(10):1403-8. doi: 10.1016/j.febslet.2012.03.016. Epub 2012 Mar 21. FEBS Lett. 2012. PMID: 22595235 Review.
Cited by
-
NF2 signaling pathway plays a pro-apoptotic role in β-adrenergic receptor stimulated cardiac myocyte apoptosis.PLoS One. 2018 Apr 30;13(4):e0196626. doi: 10.1371/journal.pone.0196626. eCollection 2018. PLoS One. 2018. PMID: 29709009 Free PMC article.
-
Hippo transducer TAZ promotes epithelial mesenchymal transition and supports pancreatic cancer progression.Oncotarget. 2015 Nov 3;6(34):35949-63. doi: 10.18632/oncotarget.5772. Oncotarget. 2015. PMID: 26416426 Free PMC article.
-
The importance of nerve microenvironment for schwannoma development.Acta Neuropathol. 2016 Aug;132(2):289-307. doi: 10.1007/s00401-016-1583-8. Epub 2016 May 28. Acta Neuropathol. 2016. PMID: 27236462 Free PMC article.
-
Splicing-Disrupting Mutations in Inherited Predisposition to Solid Pediatric Cancer.Cancers (Basel). 2022 Dec 2;14(23):5967. doi: 10.3390/cancers14235967. Cancers (Basel). 2022. PMID: 36497448 Free PMC article. Review.
-
Ocular alterations, molecular findings, and three novel pathological mutations in a series of NF2 patients.Graefes Arch Clin Exp Ophthalmol. 2019 Jul;257(7):1453-1458. doi: 10.1007/s00417-019-04348-5. Epub 2019 May 15. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 31089872
References
-
- McClatchey AI, Yap AS. Contact inhibition (of proliferation) redux. Curr Opin Cell Biol. 2012;24:685–94. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Rouleau GA, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–21. - PubMed
-
- Trofatter J, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;75:826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

