Gamma-secretase subunits associate in intracellular membrane compartments in Arabidopsis thaliana
- PMID: 24723404
- PMCID: PMC4071823
- DOI: 10.1093/jxb/eru147
Gamma-secretase subunits associate in intracellular membrane compartments in Arabidopsis thaliana
Abstract
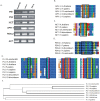
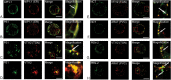
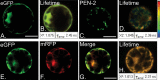
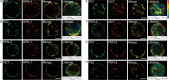
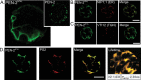
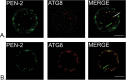
Gamma-secretase is a multisubunit complex with intramembrane proteolytic activity. In humans it was identified in genetic screens of patients suffering from familial forms of Alzheimer's disease, and since then it was shown to mediate cleavage of more than 80 substrates, including amyloid precursor protein or Notch receptor. Moreover, in animals, γ-secretase was shown to be involved in regulation of a wide range of cellular events, including cell signalling, regulation of endocytosis of membrane proteins, their trafficking, and degradation. Here we show that genes coding for γ-secretase homologues are present in plant genomes. Also, amino acid motifs crucial for γ-secretase activity are conserved in plants. Moreover, all γ-secretase subunits: PS1/PS2, APH-1, PEN-2, and NCT colocalize and interact with each other in Arabidopsis thaliana protoplasts. The intracellular localization of γ-secretase subunits in Arabidopsis protoplasts revealed a distribution in endomembrane system compartments that is consistent with data from animal studies. Together, our data may be considered as a starting point for analysis of γ-secretase in plants.
Keywords: Authophagy; complex assembly; endomembrane system; gamma-secretase; intramembrane protease; localization; presenilin; senescence; vesicular trafficking..
© The Author 2014. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures


Similar articles
-
Assembly, trafficking and function of gamma-secretase.Neurodegener Dis. 2006;3(4-5):275-83. doi: 10.1159/000095267. Neurodegener Dis. 2006. PMID: 17047368 Review.
-
Presenilin transmembrane domain 8 conserved AXXXAXXXG motifs are required for the activity of the γ-secretase complex.J Biol Chem. 2015 Mar 13;290(11):7169-84. doi: 10.1074/jbc.M114.601286. Epub 2015 Jan 22. J Biol Chem. 2015. PMID: 25614624 Free PMC article.
-
Identification of presenilin 1-selective γ-secretase inhibitors with reconstituted γ-secretase complexes.Biochemistry. 2011 Jun 7;50(22):4973-80. doi: 10.1021/bi200026m. Epub 2011 May 13. Biochemistry. 2011. PMID: 21528914
-
Membrane dynamics of γ-secretase with the anterior pharynx-defective 1B subunit.J Cell Biochem. 2021 Jan;122(1):69-85. doi: 10.1002/jcb.29832. Epub 2020 Aug 23. J Cell Biochem. 2021. PMID: 32830360
-
From presenilinase to gamma-secretase, cleave to capacitate.Curr Alzheimer Res. 2008 Apr;5(2):172-8. doi: 10.2174/156720508783954712. Curr Alzheimer Res. 2008. PMID: 18393802 Review.
Cited by
-
Non-Catalytic Roles of Presenilin Throughout Evolution.J Alzheimers Dis. 2016 Apr 12;52(4):1177-87. doi: 10.3233/JAD-150940. J Alzheimers Dis. 2016. PMID: 27079701 Free PMC article. Review.
-
ScRNA-seq reveals dark- and light-induced differentially expressed gene atlases of seedling leaves in Arachis hypogaea L.Plant Biotechnol J. 2024 Jul;22(7):1848-1866. doi: 10.1111/pbi.14306. Epub 2024 Feb 23. Plant Biotechnol J. 2024. PMID: 38391124 Free PMC article.
-
The ubiquitin ligase UBE4B regulates amyloid precursor protein ubiquitination, endosomal trafficking, and amyloid β42 generation and secretion.Mol Cell Neurosci. 2020 Oct;108:103542. doi: 10.1016/j.mcn.2020.103542. Epub 2020 Aug 22. Mol Cell Neurosci. 2020. PMID: 32841720 Free PMC article.
-
Modulation of receptor-like transmembrane kinase 1 nuclear localization by DA1 peptidases in Arabidopsis.Proc Natl Acad Sci U S A. 2022 Oct 4;119(40):e2205757119. doi: 10.1073/pnas.2205757119. Epub 2022 Sep 26. Proc Natl Acad Sci U S A. 2022. PMID: 36161927 Free PMC article.
-
Identification and Validation of Genomic Regions Associated With Charcoal Rot Resistance in Tropical Maize by Genome-Wide Association and Linkage Mapping.Front Plant Sci. 2021 Oct 8;12:726767. doi: 10.3389/fpls.2021.726767. eCollection 2021. Front Plant Sci. 2021. PMID: 34691105 Free PMC article.
References
-
- Annaert WG, Levesque L, Craessaerts K, Dierinck I, Snellings G, Westaway D, George-Hyslop PS, Cordell B, Fraser P, De Strooper B. 1999. Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons. The Journal of Cell Biology 147, 277–294 - PMC - PubMed
-
- Chakrabarty R, Banerjee R, Chung SM, Farman M, Citovsky V, Hogenhout SA, Tzfira T, Goodin M. 2007. PSITE vectors for stable integration or transient expression of autofluorescent protein fusions in plants: probing Nicotiana benthamiana-virus interactions. Molecular Plant-Microbe Interactions 20, 740–750 - PubMed
-
- Coen K, Annaert W. 2010. Presenilins: how much more than γ-secretase?! Biochemical Society Transactions 38, 1474–1478 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

