Phase I/II trial of definitive carbon ion radiotherapy for prostate cancer: evaluation of shortening of treatment period to 3 weeks
- PMID: 24722181
- PMCID: PMC4021525
- DOI: 10.1038/bjc.2014.191
Phase I/II trial of definitive carbon ion radiotherapy for prostate cancer: evaluation of shortening of treatment period to 3 weeks
Abstract
Background: The purpose of this study was to evaluate the feasibility of a new shortened 3-week treatment schedule of carbon ion radiotherapy (CIRT) for prostate cancer.
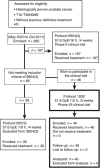
Methods: Beginning in May 2010, patients with T1b-T3bN0M0, histologically proven prostate adenocarcinoma were enrolled in the phase II trial of CIRT. Patients received 51.6 GyE in 12 fractions over 3 weeks (protocol 1002). The primary end point was defined as the incidence of late adverse events that were evaluated based on the Common Terminology Criteria for Adverse Events version 4.0. Biochemical failure was determined using the Phoenix definition (nadir +2.0 ng ml(-1)).
Results: Forty-six patients were enrolled, and all patients were included in the analysis. The number of low-, intermediate-, and high-risk patients was 12 (26%), 9 (20%), and 25 (54%), respectively. The median follow-up period of surviving patients was 32.3 months. Two patients had intercurrent death without recurrence, and the remaining 44 patients were alive at the time of this analysis. In the analysis of late toxicities, grade 1 (G1) rectal haemorrhage was observed in 3 (7%) patients. The incidence of G1 haematuria was observed in 6 (13%) patients, and G1 urinary frequency was observed in 17 (37%) patients. No ⩾G2 late toxicities were observed. In the analysis of acute toxicities, 2 (4%) patients showed G2 urinary frequency, and no other G2 acute toxicities were observed.
Conclusions: The new shortened CIRT schedule over 3 weeks was considered as feasible. The analysis of long-term outcome is warranted.
Figures
Similar articles
-
Carbon ion radiotherapy for prostate cancer with bladder invasion.BMC Urol. 2021 Aug 6;21(1):106. doi: 10.1186/s12894-021-00871-y. BMC Urol. 2021. PMID: 34362355 Free PMC article.
-
High-dose-rate brachytherapy as monotherapy for localized prostate cancer: a retrospective analysis with special focus on tolerance and chronic toxicity.Int J Radiat Oncol Biol Phys. 2003 May 1;56(1):213-20. doi: 10.1016/s0360-3016(03)00081-6. Int J Radiat Oncol Biol Phys. 2003. PMID: 12694841 Clinical Trial.
-
Multi-institutional Phase II study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities.Int J Radiat Oncol Biol Phys. 2011 Oct 1;81(2):390-6. doi: 10.1016/j.ijrobp.2010.05.027. Epub 2010 Sep 9. Int J Radiat Oncol Biol Phys. 2011. PMID: 20832180 Clinical Trial.
-
[Organ-confined prostate cancer: treatment with high doses of radioterapy (intensity modulated radiotherapy)].Actas Urol Esp. 2007 Jun;31(6):611-6. doi: 10.1016/s0210-4806(07)73697-5. Actas Urol Esp. 2007. PMID: 17896557 Review. Spanish.
-
Rotational 3D-conformal radiation therapy (conformation therapy) combined with hormone therapy for the treatment of stage B2/C prostate cancer in Japanese men.Int J Radiat Oncol Biol Phys. 2003 May 1;56(1):208-12. doi: 10.1016/s0360-3016(03)00084-1. Int J Radiat Oncol Biol Phys. 2003. PMID: 12694840 Review.
Cited by
-
Hypofractionation in prostate cancer radiotherapy: a step forward towards clinical routine.Transl Androl Urol. 2019 Dec;8(Suppl 5):S528-S532. doi: 10.21037/tau.2019.11.06. Transl Androl Urol. 2019. PMID: 32042639 Free PMC article. No abstract available.
-
Interfractional robustness of scanning carbon ion radiotherapy for prostate cancer: An analysis based on dose distribution from daily in-room CT images.J Appl Clin Med Phys. 2021 Jun;22(6):130-138. doi: 10.1002/acm2.13275. Epub 2021 May 27. J Appl Clin Med Phys. 2021. PMID: 34046997 Free PMC article.
-
Landscape of Carbon Ion Radiotherapy in Prostate Cancer: Clinical Application and Translational Research.Front Oncol. 2021 Nov 5;11:760752. doi: 10.3389/fonc.2021.760752. eCollection 2021. Front Oncol. 2021. PMID: 34804961 Free PMC article.
-
Carbon-ion radiotherapy for locally advanced cervical cancer with bladder invasion.J Radiat Res. 2016 Nov;57(6):684-690. doi: 10.1093/jrr/rrw070. Epub 2016 Jul 15. J Radiat Res. 2016. PMID: 27422932 Free PMC article.
-
Flourish of Proton and Carbon Ion Radiotherapy in China.Front Oncol. 2022 Feb 14;12:819905. doi: 10.3389/fonc.2022.819905. eCollection 2022. Front Oncol. 2022. PMID: 35237518 Free PMC article. Review.
References
-
- Akakura K, Tsujii H, Morita S, Tsuji H, Yagishita T, Isaka S, Ito H, Akaza H, Hata M, Fujime M, Harada M, Shimazaki J, Working Group for Genitourinary Tumors 2004Phase I/II clinical trials of carbon ion therapy for prostate cancer Prostate 58(3252–258.Erratum in: Prostate 2004;61(1):103. - PubMed
-
- Akimoto T, Ito K, Saitoh J, Noda SE, Harashima K, Sakurai H, Nakayama Y, Yamamoto T, Suzuki K, Nakano T, Niibe H. Acute genitourinary toxicity after high-dose-rate (HDR) brachytherapy combined with hypofractionated external-beam radiation therapy for localized prostate cancer: correlation between the urethral dose in HDR brachytherapy and the severity of acute genitourinary toxicity. Int J Radiat Oncol Biol Phys. 2005;63 (2:463–471. - PubMed
-
- Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Billiet I, Torecilla JL, Pfeffer R, Cutajar CL, Van der Kwast T, Collette L. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11 (11:1066–1073. - PubMed
-
- Cahlon O, Zelefsky MJ, Shippy A, Chan H, Fuks Z, Yamada Y, Hunt M, Greenstein S, Amols H. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys. 2008;71 (2:330–337. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical