Early forming label-retaining muscle stem cells require p27kip1 for maintenance of the primitive state
- PMID: 24715455
- PMCID: PMC3978835
- DOI: 10.1242/dev.100842
Early forming label-retaining muscle stem cells require p27kip1 for maintenance of the primitive state
Abstract
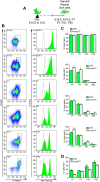
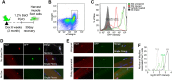
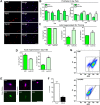
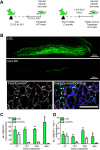
Across different niches, subsets of highly functional stem cells are maintained in a relatively dormant rather than proliferative state. Our understanding of proliferative dynamics in tissue-specific stem cells during conditions of increased tissue turnover remains limited. Using a TetO-H2B-GFP reporter of proliferative history, we identify skeletal muscle stem cell, or satellite cells, that retain (LRC) or lose (nonLRC) the H2B-GFP label. We show in mice that LRCs and nonLRCs are formed at birth and persist during postnatal growth and adult muscle repair. Functionally, LRCs and nonLRCs are born equivalent and transition during postnatal maturation into distinct and hierarchically organized subsets. Adult LRCs give rise to LRCs and nonLRCs; the former are able to self-renew, whereas the latter are restricted to differentiation. Expression analysis revealed the CIP/KIP family members p21(cip1) (Cdkn1a) and p27(kip1) (Cdkn1b) to be expressed at higher levels in LRCs. In accordance with a crucial role in LRC fate, loss of p27(kip1) promoted proliferation and differentiation of LRCs in vitro and impaired satellite cell self-renewal after muscle injury. By contrast, loss of p21(cip1) only affected nonLRCs, in which myogenic commitment was inhibited. Our results provide evidence that restriction of self-renewal potential to LRCs is established early in life and is maintained during increased tissue turnover through the cell cycle inhibitor p27(kip1). They also reveal the differential role of CIP/KIP family members at discrete steps within the stem cell hierarchy.
Keywords: Mouse; Muscle; Quiescence; Regeneration; Satellite cell; Self-renewal; Stem cell.
Figures
Similar articles
-
Roles for p21waf1/cip1 and p27kip1 during the adaptation response to massive intestinal resection.Am J Physiol Gastrointest Liver Physiol. 2006 May;290(5):G933-41. doi: 10.1152/ajpgi.00235.2005. Epub 2005 Dec 1. Am J Physiol Gastrointest Liver Physiol. 2006. PMID: 16322092
-
CDK inhibitors, p21(Cip1) and p27(Kip1), participate in cell cycle exit of mammalian cardiomyocytes.Biochem Biophys Res Commun. 2014 Jan 17;443(3):1105-9. doi: 10.1016/j.bbrc.2013.12.109. Epub 2013 Dec 28. Biochem Biophys Res Commun. 2014. PMID: 24380855
-
p27(Kip1) and p21(Cip1)-independent proliferative inhibition of vascular smooth muscle cells cultured in type-I collagen matrix honeycombs.Microvasc Res. 2016 Jan;103:36-40. doi: 10.1016/j.mvr.2015.10.003. Epub 2015 Oct 29. Microvasc Res. 2016. PMID: 26522285
-
[Lineage-switching by pluripotent cells derived from adults].J Soc Biol. 2001;195(1):39-46. J Soc Biol. 2001. PMID: 11530498 Review. French.
-
Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors.Cell Cycle. 2010 Jun 15;9(12):2342-52. doi: 10.4161/cc.9.12.11988. Epub 2010 Jun 15. Cell Cycle. 2010. PMID: 20519948 Free PMC article. Review.
Cited by
-
Insights into muscle stem cell dynamics during postnatal development.FEBS J. 2022 May;289(10):2710-2722. doi: 10.1111/febs.15856. Epub 2021 Apr 18. FEBS J. 2022. PMID: 33811430 Free PMC article. Review.
-
Wnt4 from the Niche Controls the Mechano-Properties and Quiescent State of Muscle Stem Cells.Cell Stem Cell. 2019 Nov 7;25(5):654-665.e4. doi: 10.1016/j.stem.2019.08.007. Epub 2019 Sep 5. Cell Stem Cell. 2019. PMID: 31495781 Free PMC article.
-
The AMPK/p27Kip1 Axis Regulates Autophagy/Apoptosis Decisions in Aged Skeletal Muscle Stem Cells.Stem Cell Reports. 2018 Aug 14;11(2):425-439. doi: 10.1016/j.stemcr.2018.06.014. Epub 2018 Jul 19. Stem Cell Reports. 2018. PMID: 30033086 Free PMC article.
-
Myogenic Precursor Cells Show Faster Activation and Enhanced Differentiation in a Male Mouse Model Selected for Advanced Endurance Exercise Performance.Cells. 2022 Mar 16;11(6):1001. doi: 10.3390/cells11061001. Cells. 2022. PMID: 35326452 Free PMC article.
-
Aging induces aberrant state transition kinetics in murine muscle stem cells.Development. 2020 May 5;147(9):dev183855. doi: 10.1242/dev.183855. Development. 2020. PMID: 32198156 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

