CCM1 and the second life of proteins in adhesion complexes
- PMID: 24714220
- PMCID: PMC4049860
- DOI: 10.4161/cam.28437
CCM1 and the second life of proteins in adhesion complexes
Abstract
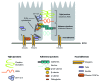
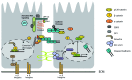
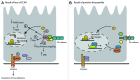
It is well recognized that a number of proteins present within adhesion complexes perform discrete signaling functions outside these adhesion complexes, including transcriptional control. In this respect, β-catenin is a well-known example of an adhesion protein present both in cadherin complexes and in the nucleus where it regulates the TCF transcription factor. Here we discuss nuclear functions of adhesion complex proteins with a special focus on the CCM-1/KRIT-1 protein, which may turn out to be yet another adhesion complex protein with a second life.
Keywords: CCM1/Krit-1; Rac Rho; adhesion signaling; nuclear translocation; small GTPases; transcription.
Figures

Similar articles
-
Rap1 and its effector KRIT1/CCM1 regulate beta-catenin signaling.Dis Model Mech. 2010 Jan-Feb;3(1-2):73-83. doi: 10.1242/dmm.003293. Epub 2009 Dec 9. Dis Model Mech. 2010. PMID: 20007487 Free PMC article.
-
CCM1 regulates vascular-lumen organization by inducing endothelial polarity.J Cell Sci. 2010 Apr 1;123(Pt 7):1073-80. doi: 10.1242/jcs.059329. J Cell Sci. 2010. PMID: 20332120
-
Wnt-5a-CKI{alpha} signaling promotes {beta}-catenin/E-cadherin complex formation and intercellular adhesion in human breast epithelial cells.J Biol Chem. 2009 Apr 17;284(16):10968-79. doi: 10.1074/jbc.M804923200. Epub 2009 Feb 25. J Biol Chem. 2009. PMID: 19244247 Free PMC article.
-
Recent insights into cerebral cavernous malformations: a complex jigsaw puzzle under construction.FEBS J. 2010 Mar;277(5):1084-96. doi: 10.1111/j.1742-4658.2009.07537.x. Epub 2010 Jan 22. FEBS J. 2010. PMID: 20096036 Free PMC article. Review.
-
Coordinating Rho and Rac: the regulation of Rho GTPase signaling and cadherin junctions.Prog Mol Biol Transl Sci. 2013;116:49-68. doi: 10.1016/B978-0-12-394311-8.00003-0. Prog Mol Biol Transl Sci. 2013. PMID: 23481190 Review.
Cited by
-
Molecular analysis of gastric cancer identifies genomic markers of drug sensitivity in Asian gastric cancer.J Cancer. 2018 Jul 30;9(16):2973-2980. doi: 10.7150/jca.25506. eCollection 2018. J Cancer. 2018. PMID: 30123366 Free PMC article.
-
Molecular Genetic Features of Cerebral Cavernous Malformations (CCM) Patients: An Overall View from Genes to Endothelial Cells.Cells. 2021 Mar 22;10(3):704. doi: 10.3390/cells10030704. Cells. 2021. PMID: 33810005 Free PMC article. Review.
-
Cancer-secreted exosomal miR-21-5p induces angiogenesis and vascular permeability by targeting KRIT1.Cell Death Dis. 2021 Jun 4;12(6):576. doi: 10.1038/s41419-021-03803-8. Cell Death Dis. 2021. PMID: 34088891 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
