RSV-encoded NS2 promotes epithelial cell shedding and distal airway obstruction
- PMID: 24713657
- PMCID: PMC4001550
- DOI: 10.1172/JCI72948
RSV-encoded NS2 promotes epithelial cell shedding and distal airway obstruction
Abstract
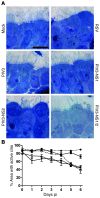
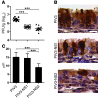
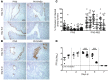
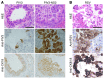
Respiratory syncytial virus (RSV) infection is the major cause of bronchiolitis in young children. The factors that contribute to the increased propensity of RSV-induced distal airway disease compared with other commonly encountered respiratory viruses remain unclear. Here, we identified the RSV-encoded nonstructural 2 (NS2) protein as a viral genetic determinant for initiating RSV-induced distal airway obstruction. Infection of human cartilaginous airway epithelium (HAE) and a hamster model of disease with recombinant respiratory viruses revealed that NS2 promotes shedding of infected epithelial cells, resulting in two consequences of virus infection. First, epithelial cell shedding accelerated the reduction of virus titers, presumably by clearing virus-infected cells from airway mucosa. Second, epithelial cells shedding into the narrow-diameter bronchiolar airway lumens resulted in rapid accumulation of detached, pleomorphic epithelial cells, leading to acute distal airway obstruction. Together, these data indicate that RSV infection of the airway epithelium, via the action of NS2, promotes epithelial cell shedding, which not only accelerates viral clearance but also contributes to acute obstruction of the distal airways. Our results identify RSV NS2 as a contributing factor for the enhanced propensity of RSV to cause severe airway disease in young children and suggest NS2 as a potential therapeutic target for reducing the severity of distal airway disease.
Figures

Similar articles
-
Respiratory syncytial virus nonstructural protein 2 specifically inhibits type I interferon signal transduction.Virology. 2006 Jan 20;344(2):328-39. doi: 10.1016/j.virol.2005.09.009. Epub 2005 Oct 10. Virology. 2006. PMID: 16216295
-
Enhanced susceptibility of pediatric airway epithelium to respiratory syncytial virus infection.J Clin Invest. 2024 Nov 1;134(21):e185689. doi: 10.1172/JCI185689. J Clin Invest. 2024. PMID: 39484717 Free PMC article.
-
Respiratory Syncytial Virus and Human Metapneumovirus Infections in Three-Dimensional Human Airway Tissues Expose an Interesting Dichotomy in Viral Replication, Spread, and Inhibition by Neutralizing Antibodies.J Virol. 2020 Sep 29;94(20):e01068-20. doi: 10.1128/JVI.01068-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32759319 Free PMC article.
-
Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis.J Pathol. 2015 Jan;235(2):266-76. doi: 10.1002/path.4462. J Pathol. 2015. PMID: 25302625 Free PMC article. Review.
-
Respiratory syncytial virus interaction with human airway epithelium.Trends Microbiol. 2013 May;21(5):238-44. doi: 10.1016/j.tim.2013.02.004. Epub 2013 Mar 22. Trends Microbiol. 2013. PMID: 23523320 Review.
Cited by
-
An overview on the RSV-mediated mechanisms in the onset of non-allergic asthma.Front Pediatr. 2022 Sep 20;10:998296. doi: 10.3389/fped.2022.998296. eCollection 2022. Front Pediatr. 2022. PMID: 36204661 Free PMC article. Review.
-
Advanced Live Attenuated Vaccines for the Prevention of Respiratory Syncytial Virus Infections in Young Children.J Infect Dis. 2020 Jun 16;222(1):4-6. doi: 10.1093/infdis/jiz409. J Infect Dis. 2020. PMID: 31549154 Free PMC article. No abstract available.
-
The Multifaceted Roles of Autophagy in Infectious, Obstructive, and Malignant Airway Diseases.Biomedicines. 2022 Aug 11;10(8):1944. doi: 10.3390/biomedicines10081944. Biomedicines. 2022. PMID: 36009490 Free PMC article. Review.
-
Predictors of prolonged length of stay in adult patients with respiratory syncytial virus infections - a multi-center historical cohort study.Front Microbiol. 2024 Apr 4;15:1385439. doi: 10.3389/fmicb.2024.1385439. eCollection 2024. Front Microbiol. 2024. PMID: 38638901 Free PMC article.
-
Human T cells efficiently control RSV infection.JCI Insight. 2023 Jun 8;8(11):e168110. doi: 10.1172/jci.insight.168110. JCI Insight. 2023. PMID: 37159271 Free PMC article.
References
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

