Dramatic differences in the response of macrophages from B2 and B19 MHC-defined haplotypes to interferon gamma and polyinosinic:polycytidylic acid stimulation
- PMID: 24706959
- PMCID: PMC7107093
- DOI: 10.3382/ps.2013-03511
Dramatic differences in the response of macrophages from B2 and B19 MHC-defined haplotypes to interferon gamma and polyinosinic:polycytidylic acid stimulation
Abstract
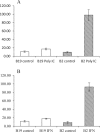
The chicken MHC has been associated with disease resistance, though the mechanisms are not understood. The functions of macrophages, critical to both innate and acquired immunity, were compared between the more infectious bronchitis virus-resistant B2 and the more infectious bronchitis virus-susceptible B19 lines. In vivo peripheral blood concentrations of monocytes were similar in B2 or B19 homozygous haplotypes. Peripheral blood-derived macrophages were stimulated with poly I:C, simulating an RNA virus, or IFNγ, a cytokine at the interface of innate and adaptive immunity. Not only did B2-derived peripheral monocytes differentiate into macrophages more readily than the B19 monocytes, but as determined by NO production, macrophages from B2 and B2 on B19 genetic background chicks were also significantly more responsive to either stimulant. In conclusion, the correlation with resistance to illness following viral infection may be directly linked to a more vigorous innate immune response.
Keywords: disease resistance; infectious bronchitis virus; innate immunity; macrophage; monocyte.
Figures


Similar articles
-
Association of the chicken MHC B haplotypes with resistance to avian coronavirus.Dev Comp Immunol. 2013 Apr;39(4):430-7. doi: 10.1016/j.dci.2012.10.006. Epub 2012 Nov 23. Dev Comp Immunol. 2013. PMID: 23178407 Free PMC article.
-
Effects of Chicken MHC Haplotype on Resistance to Distantly Related Infectious Bronchitis Viruses.Avian Dis. 2019 Jun 1;63(2):310-317. doi: 10.1637/11989-103118-Reg.1. Avian Dis. 2019. PMID: 31251532
-
Cytokine Responses in Tracheas from Major Histocompatibility Complex Congenic Chicken Lines with Distinct Susceptibilities to Infectious Bronchitis Virus.Avian Dis. 2020 Mar;64(1):36-45. doi: 10.1637/0005-2086-64.1.36. Avian Dis. 2020. PMID: 32267123
-
Immune Responses to Virulent and Vaccine Strains of Infectious Bronchitis Viruses in Chickens.Viral Immunol. 2015 Nov;28(9):478-88. doi: 10.1089/vim.2015.0027. Epub 2015 Aug 24. Viral Immunol. 2015. PMID: 26301315 Review.
-
[Advance in association studies of major histocompatibility complex (MHC) gene polymorphisms with traits of resistance against infectious disease in chickens].Yi Chuan. 2006 Jul;28(7):893-8. Yi Chuan. 2006. PMID: 16825180 Review. Chinese.
Cited by
-
ALV-J infection induces chicken monocyte death accompanied with the production of IL-1β and IL-18.Oncotarget. 2017 Oct 13;8(59):99889-99900. doi: 10.18632/oncotarget.21906. eCollection 2017 Nov 21. Oncotarget. 2017. PMID: 29245947 Free PMC article.
-
ALV-J strain SCAU-HN06 induces innate immune responses in chicken primary monocyte-derived macrophages.Poult Sci. 2017 Jan 1;96(1):42-50. doi: 10.3382/ps/pew229. Epub 2016 Aug 2. Poult Sci. 2017. PMID: 27486255 Free PMC article.
-
Chondronecrosis with osteomyelitis in broilers: further defining lameness-inducing models with wire or litter flooring to evaluate protection with organic trace minerals.Poult Sci. 2020 Nov;99(11):5422-5429. doi: 10.1016/j.psj.2020.08.027. Epub 2020 Aug 31. Poult Sci. 2020. PMID: 33142459 Free PMC article.
-
Deciphering desirable immune responses from disease models with resistant and susceptible chickens.Poult Sci. 2019 Apr 1;98(4):1634-1642. doi: 10.3382/ps/pey535. Poult Sci. 2019. PMID: 30534980 Free PMC article. Review.
-
Brief review of the chicken Major Histocompatibility Complex: the genes, their distribution on chromosome 16, and their contributions to disease resistance.Poult Sci. 2016 Feb;95(2):375-92. doi: 10.3382/ps/pev379. Epub 2016 Jan 6. Poult Sci. 2016. PMID: 26740135 Free PMC article. Review.
References
-
- Bacon L.D., Hunter D.B., Zhang H.M., Brand K., Etches R. Retrospective evidence that the MHC (B haplotype) of chickens influences genetic resistance to attenuated infectious bronchitis vaccine strains in chickens. Avian Pathol. 2004;33:605–609. 15763730. - PubMed
-
- Bayyari G.R., Huff W.E., Rath N.C., Balog J.M., Newberry L.A., Villines J.D., Skeeles J.K., Anthony N.B., Nestor K.E. Effect of the genetic selection of turkeys for increased body weight and egg production on immune and physiological responses. Poult. Sci. 1997;76:289–296. 9057208. - PubMed
-
- Bearse G.E., McClary C.F., Miller M.W. The results of eight years’ selection for disease resistance and susceptibility in White Leghorns. Poult. Sci. 1939;18:400–401.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

