CCR2 deficiency promotes exacerbated chronic erosive neutrophil-dominated chikungunya virus arthritis
- PMID: 24696480
- PMCID: PMC4054367
- DOI: 10.1128/JVI.03364-13
CCR2 deficiency promotes exacerbated chronic erosive neutrophil-dominated chikungunya virus arthritis
Abstract
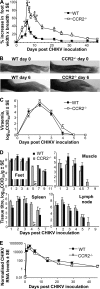
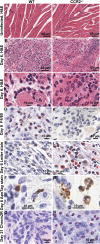
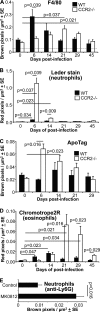
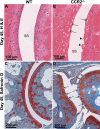
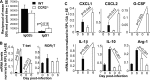
Chikungunya virus (CHIKV) is a member of a globally distributed group of arthritogenic alphaviruses that cause weeks to months of debilitating polyarthritis/arthralgia, which is often poorly managed with current treatments. Arthritic disease is usually characterized by high levels of the chemokine CCL2 and a prodigious monocyte/macrophage infiltrate. Several inhibitors of CCL2 and its receptor CCR2 are in development and may find application for treatment of certain inflammatory conditions, including autoimmune and viral arthritides. Here we used CCR2(-/-) mice to determine the effect of CCR2 deficiency on CHIKV infection and arthritis. Although there were no significant changes in viral load or RNA persistence and only marginal changes in antiviral immunity, arthritic disease was substantially increased and prolonged in CCR2(-/-) mice compared to wild-type mice. The monocyte/macrophage infiltrate was replaced in CCR2(-/-) mice by a severe neutrophil (followed by an eosinophil) infiltrate and was associated with changes in the expression levels of multiple inflammatory mediators (including CXCL1, CXCL2, granulocyte colony-stimulating factor [G-CSF], interleukin-1β [IL-1β], and IL-10). The loss of anti-inflammatory macrophages and their activities (e.g., efferocytosis) was also implicated in exacerbated inflammation. Clear evidence of cartilage damage was also seen in CHIKV-infected CCR2(-/-) mice, a feature not normally associated with alphaviral arthritides. Although recruitment of CCR2(+) monocytes/macrophages can contribute to inflammation, it also appears to be critical for preventing excessive pathology and resolving inflammation following alphavirus infection. Caution might thus be warranted when considering therapeutic targeting of CCR2/CCL2 for the treatment of alphaviral arthritides.
Importance: Here we describe the first analysis of viral arthritis in mice deficient for the chemokine receptor CCR2. CCR2 is thought to be central to the monocyte/macrophage-dominated inflammatory arthritic infiltrates seen after infection with arthritogenic alphaviruses such as chikungunya virus. Surprisingly, the viral arthritis caused by chikungunya virus in CCR2-deficient mice was more severe, prolonged, and erosive and was neutrophil dominated, with viral replication and persistence not being significantly affected. Monocytes/macrophages recruited by CCL2 thus also appear to be important for both preventing even worse pathology mediated by neutrophils and promoting resolution of inflammation. Caution might thus be warranted when considering the use of therapeutic agents that target CCR2/CCL2 or inflammatory monocytes/macrophages for the treatment of alphaviral (and perhaps other viral) arthritides. Individuals with diminished CCR2 responses (due to drug treatment or other reasons) may also be at risk of exacerbated arthritic disease following alphaviral infection.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Essential role of the CCL2-CCR2 axis in Mayaro virus-induced disease.J Virol. 2024 Jan 23;98(1):e0110223. doi: 10.1128/jvi.01102-23. Epub 2024 Jan 3. J Virol. 2024. PMID: 38169294 Free PMC article.
-
Inflammatory monocytes mediate control of acute alphavirus infection in mice.PLoS Pathog. 2017 Dec 15;13(12):e1006748. doi: 10.1371/journal.ppat.1006748. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29244871 Free PMC article.
-
Macrophage migration inhibitory factor receptor CD74 mediates alphavirus-induced arthritis and myositis in murine models of alphavirus infection.Arthritis Rheum. 2013 Oct;65(10):2724-36. doi: 10.1002/art.38090. Arthritis Rheum. 2013. PMID: 23896945 Free PMC article.
-
Arthritogenic Alphavirus-Induced Immunopathology and Targeting Host Inflammation as A Therapeutic Strategy for Alphaviral Disease.Viruses. 2019 Mar 22;11(3):290. doi: 10.3390/v11030290. Viruses. 2019. PMID: 30909385 Free PMC article. Review.
-
Chikungunya Virus-Induced Arthritis: Role of Host and Viral Factors in the Pathogenesis.Viral Immunol. 2017 Dec;30(10):691-702. doi: 10.1089/vim.2017.0052. Epub 2017 Sep 14. Viral Immunol. 2017. PMID: 28910194 Review.
Cited by
-
Loss of CCR2 signaling alters leukocyte recruitment and exacerbates γ-herpesvirus-induced pneumonitis and fibrosis following bone marrow transplantation.Am J Physiol Lung Cell Mol Physiol. 2016 Sep 1;311(3):L611-27. doi: 10.1152/ajplung.00193.2016. Epub 2016 Jul 22. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27448666 Free PMC article.
-
CCR2 Regulates Vaccine-Induced Mucosal T-Cell Memory to Influenza A Virus.J Virol. 2021 Jul 12;95(15):e0053021. doi: 10.1128/JVI.00530-21. Epub 2021 Jul 12. J Virol. 2021. PMID: 33952647 Free PMC article.
-
Cellular and Molecular Immune Response to Chikungunya Virus Infection.Front Cell Infect Microbiol. 2018 Oct 10;8:345. doi: 10.3389/fcimb.2018.00345. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30364124 Free PMC article. Review.
-
Mutating chikungunya virus non-structural protein produces potent live-attenuated vaccine candidate.EMBO Mol Med. 2019 Jun;11(6):e10092. doi: 10.15252/emmm.201810092. EMBO Mol Med. 2019. PMID: 31015278 Free PMC article.
-
Complex Roles of Neutrophils during Arboviral Infections.Cells. 2021 May 26;10(6):1324. doi: 10.3390/cells10061324. Cells. 2021. PMID: 34073501 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

