Siglec-G-CD24 axis controls the severity of graft-versus-host disease in mice
- PMID: 24695850
- PMCID: PMC4041170
- DOI: 10.1182/blood-2013-12-545335
Siglec-G-CD24 axis controls the severity of graft-versus-host disease in mice
Abstract
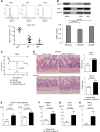
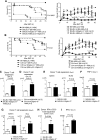
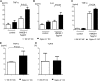
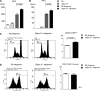
Activation of sialic-acid-binding immunoglobulin-like lectin-G (Siglec-G) by noninfectious damage-associated molecular patterns controls innate immune responses. However, whether it also regulates T-cell-mediated adaptive immune responses is not known. Graft-versus-host reaction is a robust adaptive immune response caused by allogeneic hematopoietic cell transplantation that have been activated by antigen-presenting cells (APCs) in the context of damaged host tissues following allogeneic hematopoietic cell transplantation. The role of infectious and noninfectious pattern recognition receptor-mediated activation in the induction and aggravation of graft-versus-host disease (GVHD) is being increasingly appreciated. But the role of pathways that control innate immune responses to noninfectious stimuli in modulating GVHD has heretofore not been recognized. We report that Siglec-G expression on host APCs, specifically on hematopoietic cells, negatively regulates GVHD in multiple clinically relevant murine models. Mechanistic studies with various relevant Siglec-G and CD24 knockout mice and chimeric animals, along with rescue experiments with novel CD24 fusion protein demonstrate that enhancing the interaction between Siglec-G on host APCs with CD24 on donor T cells attenuates GVHD. Taken together, our data demonstrate that Siglec-G-CD24 axis, controls the severity of GVHD and suggest that enhancing this interaction may represent a novel strategy for mitigating GVHD.
© 2014 by The American Society of Hematology.
Figures


Comment in
-
Siglec-G signaling DAMPens GVHD.Blood. 2014 May 29;123(22):3376-7. doi: 10.1182/blood-2014-04-568832. Blood. 2014. PMID: 24876528 No abstract available.
Similar articles
-
Siglec-G signaling DAMPens GVHD.Blood. 2014 May 29;123(22):3376-7. doi: 10.1182/blood-2014-04-568832. Blood. 2014. PMID: 24876528 No abstract available.
-
CD24 and Siglec-10 selectively repress tissue damage-induced immune responses.Science. 2009 Mar 27;323(5922):1722-5. doi: 10.1126/science.1168988. Epub 2009 Mar 5. Science. 2009. PMID: 19264983 Free PMC article.
-
STING negatively regulates allogeneic T-cell responses by constraining antigen-presenting cell function.Cell Mol Immunol. 2021 Mar;18(3):632-643. doi: 10.1038/s41423-020-00611-6. Epub 2021 Jan 26. Cell Mol Immunol. 2021. PMID: 33500563 Free PMC article.
-
The primacy of gastrointestinal tract antigen-presenting cells in lethal graft-versus-host disease.Blood. 2019 Dec 12;134(24):2139-2148. doi: 10.1182/blood.2019000823. Blood. 2019. PMID: 31697827 Free PMC article. Review.
-
Siglec-G/10 in self-nonself discrimination of innate and adaptive immunity.Glycobiology. 2014 Sep;24(9):800-6. doi: 10.1093/glycob/cwu068. Epub 2014 Jul 4. Glycobiology. 2014. PMID: 24996822 Free PMC article. Review.
Cited by
-
Immunopathology and biology-based treatment of steroid-refractory graft-versus-host disease.Blood. 2020 Jul 23;136(4):429-440. doi: 10.1182/blood.2019000953. Blood. 2020. PMID: 32526035 Free PMC article. Review.
-
The Fab Fragment of a Human Anti-Siglec-9 Monoclonal Antibody Suppresses LPS-Induced Inflammatory Responses in Human Macrophages.Front Immunol. 2016 Dec 26;7:649. doi: 10.3389/fimmu.2016.00649. eCollection 2016. Front Immunol. 2016. PMID: 28082984 Free PMC article.
-
Inflammation, immunity and potential target therapy of SARS-COV-2: A total scale analysis review.Food Chem Toxicol. 2021 Apr;150:112087. doi: 10.1016/j.fct.2021.112087. Epub 2021 Feb 25. Food Chem Toxicol. 2021. PMID: 33640537 Free PMC article. Review.
-
Various forms of tissue damage and danger signals following hematopoietic stem-cell transplantation.Front Immunol. 2015 Jan 28;6:14. doi: 10.3389/fimmu.2015.00014. eCollection 2015. Front Immunol. 2015. PMID: 25674088 Free PMC article. Review.
-
GVHD Prophylaxis 2020.Front Immunol. 2021 Apr 7;12:605726. doi: 10.3389/fimmu.2021.605726. eCollection 2021. Front Immunol. 2021. PMID: 33897681 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

