Influence of enteric infections on response to oral poliovirus vaccine: a systematic review and meta-analysis
- PMID: 24688069
- PMCID: PMC4136801
- DOI: 10.1093/infdis/jiu182
Influence of enteric infections on response to oral poliovirus vaccine: a systematic review and meta-analysis
Abstract
Background: The impaired immunogenicity of oral poliovirus vaccine (OPV) in low-income countries has been apparent since the early field trials of this vaccine. Infection with enteropathogens at the time of vaccination may contribute to this phenomenon. However, the relative influence of these infections on OPV performance remains uncertain.
Methods: We conducted a systematic review to examine the impact of concurrent enteric infections on OPV response. Using random-effects models, we assessed the effects of nonpolio enteroviruses (NPEVs) and diarrhea on the odds of seroconversion and/or vaccine virus shedding.
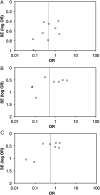
Results: We identified 25 trials in which OPV outcomes were compared according to the presence or absence of enteric infections, the majority of which (n = 17) reported only on NPEVs. Concurrent NPEVs significantly reduced the odds of per-dose seroconversion for type 1 poliovirus (odds ratio [OR] 0.44, 95% confidence interval 0.23-0.84), but not type 2 (OR 0.53 [0.19-1.46]) or type 3 (OR 0.56 [0.27-1.12]). A similar reduction, significant for type 1 poliovirus (OR 0.50 [0.28-0.89]), was observed in the odds of vaccine virus shedding among NPEV-infected individuals. Concurrent diarrhea significantly inhibited per-dose seroconversion overall (OR 0.61 [0.38-0.87]).
Conclusions: Our findings are consistent with an inhibitory effect of concurrent enteric infections on OPV response.
Keywords: diarrhea; enterovirus; immunogenicity; interference; oral poliovirus vaccine.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Figures




Similar articles
-
Influence of Nonpolio Enteroviruses and the Bacterial Gut Microbiota on Oral Poliovirus Vaccine Response: A Study from South India.J Infect Dis. 2019 Apr 8;219(8):1178-1186. doi: 10.1093/infdis/jiy568. J Infect Dis. 2019. PMID: 30247561 Free PMC article.
-
Vaccine schedules and the effect on humoral and intestinal immunity against poliovirus: a systematic review and network meta-analysis.Lancet Infect Dis. 2019 Oct;19(10):1121-1128. doi: 10.1016/S1473-3099(19)30301-9. Epub 2019 Jul 23. Lancet Infect Dis. 2019. PMID: 31350192
-
Impact of enterovirus and other enteric pathogens on oral polio and rotavirus vaccine performance in Bangladeshi infants.Vaccine. 2016 Jun 8;34(27):3068-3075. doi: 10.1016/j.vaccine.2016.04.080. Epub 2016 May 3. Vaccine. 2016. PMID: 27154394 Free PMC article. Clinical Trial.
-
Oral and inactivated poliovirus vaccines in the newborn: a review.Vaccine. 2013 May 17;31(21):2517-24. doi: 10.1016/j.vaccine.2012.06.020. Epub 2012 Jun 20. Vaccine. 2013. PMID: 22728224 Free PMC article. Review.
-
Global eradication of poliomyelitis: reinvent the wheel or use existing options effectively?Public Health Rev. 1993-1994;21(1-2):143-50. Public Health Rev. 1993. PMID: 8041881 Review.
Cited by
-
Poliovirus antibodies following two rounds of campaigns with a type 2 novel oral poliovirus vaccine in Liberia: a clustered, population-based seroprevalence survey.Lancet Glob Health. 2023 Jun;11(6):e917-e923. doi: 10.1016/S2214-109X(23)00116-X. Lancet Glob Health. 2023. PMID: 37202026 Free PMC article.
-
Gut microbiota and synbiotic foods: Unveiling the relationship in COVID-19 perspective.Food Sci Nutr. 2023 Jan 11;11(3):1166-1177. doi: 10.1002/fsn3.3162. eCollection 2023 Mar. Food Sci Nutr. 2023. PMID: 36911846 Free PMC article. Review.
-
The Origins and Risk Factors for Serotype-2 Vaccine-Derived Poliovirus Emergences in Africa During 2016-2019.J Infect Dis. 2023 Jun 28;228(1):80-88. doi: 10.1093/infdis/jiad004. J Infect Dis. 2023. PMID: 36630295 Free PMC article.
-
Nanoparticle- and Microparticle-Based Vaccines against Orbiviruses of Veterinary Importance.Vaccines (Basel). 2022 Jul 14;10(7):1124. doi: 10.3390/vaccines10071124. Vaccines (Basel). 2022. PMID: 35891288 Free PMC article. Review.
-
Immunity to enteric viruses.Immunity. 2022 May 10;55(5):800-818. doi: 10.1016/j.immuni.2022.04.007. Immunity. 2022. PMID: 35545029 Free PMC article. Review.
References
-
- Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13:926–39. - PubMed
-
- Sabin AB, Michaels RH, Spigland I, Pelon W, Rhim JS, Wehr RE. Community-wide use of oral poliovirus vaccine. Effectiveness of the Cincinnati program. Am J Dis Child. 1961;101:546–67. - PubMed
-
- Global Polio Eradication Initiative. Polio eradication and endgame strategic plan (2013–2018) http://www.polioeradication.org/resourcelibrary/strategyandwork.aspx. Accessed 3 September 2013.
-
- Su-Arehawaratana P, Singharaj P, Taylor DN, et al. Safety and immunogenicity of different immunization regimens of CVD 103-HgR live oral cholera vaccine in soldiers and civilians in Thailand. J Infect Dis. 1992;165:1042–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

