In search of antiaging modalities: evaluation of mTOR- and ROS/DNA damage-signaling by cytometry
- PMID: 24677687
- PMCID: PMC4080725
- DOI: 10.1002/cyto.a.22452
In search of antiaging modalities: evaluation of mTOR- and ROS/DNA damage-signaling by cytometry
Abstract
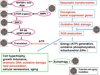
This review presents the evidence in support of the IGF-1/mTOR/S6K1 signaling as the primary factor contributing to aging and cellular senescence. Reviewed are also specific interactions between mTOR/S6K1 and ROS-DNA damage signaling pathways. Outlined are critical sites along these pathways, including autophagy, as targets for potential antiaging (gero-suppressive) and/or chemopreventive agents. Presented are applications of flow- and laser scanning- cytometry utilizing phospho-specific Abs, to monitor activation along these pathways in response to the reported antiaging drugs rapamycin, metformin, berberine, resveratrol, vitamin D3, 2-deoxyglucose, and acetylsalicylic acid. Specifically, effectiveness of these agents to attenuate the level of constitutive mTOR signaling was tested by cytometry and confirmed by Western blotting through measuring phosphorylation of the mTOR-downstream targets including ribosomal protein S6. The ratiometric analysis of phosphorylated to total protein along the mTOR pathway offers a useful parameter reporting the effects of gero-suppressive agents. In parallel, their ability to suppress the level of constitutive DNA damage signaling induced by endogenous ROS was measured. While the primary target of each of these agents may be different the data obtained on several human cancer cell lines, WI-38 fibroblasts and normal lymphocytes suggest common downstream mechanism in which the decline in mTOR/S6K1 signaling and translation rate is coupled with a reduction of oxidative phosphorylation and ROS that leads to decreased oxidative DNA damage. The combined assessment of constitutive γH2AX expression, mitochondrial activity (ROS, ΔΨm), and mTOR signaling provides an adequate gamut of cell responses to test effectiveness of gero-suppressive agents. Described is also an in vitro model of induction of cellular senescence by persistent replication stress, its quantitative analysis by laser scanning cytometry, and application to detect the property of the studied agents to attenuate the induction of senescence. Discussed is cytometric analysis of cell size and heterogeneity of size as a potential biomarker used to asses gero-suppressive agents and longevity.
Keywords: DNA damage signaling; H2AX phosphorylation; berberine; cell size; cellular senescence; mitochondria; red cell distribution width; replication stress; ribosomal protein S6 phosphorylation; translation.
© 2014 International Society for Advancement of Cytometry.
Figures







Similar articles
-
Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling.Aging (Albany NY). 2012 Dec;4(12):952-65. doi: 10.18632/aging.100521. Aging (Albany NY). 2012. PMID: 23363784 Free PMC article.
-
Berberine suppresses gero-conversion from cell cycle arrest to senescence.Aging (Albany NY). 2013 Aug;5(8):623-36. doi: 10.18632/aging.100593. Aging (Albany NY). 2013. PMID: 23974852 Free PMC article.
-
Autophagy impairment induces premature senescence in primary human fibroblasts.PLoS One. 2011;6(8):e23367. doi: 10.1371/journal.pone.0023367. Epub 2011 Aug 8. PLoS One. 2011. PMID: 21858089 Free PMC article.
-
Aberrant mTOR activation in senescence and aging: A mitochondrial stress response?Exp Gerontol. 2015 Aug;68:66-70. doi: 10.1016/j.exger.2014.11.004. Epub 2014 Nov 6. Exp Gerontol. 2015. PMID: 25449851 Free PMC article. Review.
-
mTOR-regulated senescence and autophagy during reprogramming of somatic cells to pluripotency: a roadmap from energy metabolism to stem cell renewal and aging.Cell Cycle. 2011 Nov 1;10(21):3658-77. doi: 10.4161/cc.10.21.18128. Epub 2011 Nov 1. Cell Cycle. 2011. PMID: 22052357 Review.
Cited by
-
Synergistic cell death in FLT3-ITD positive acute myeloid leukemia by combined treatment with metformin and 6-benzylthioinosine.Leuk Res. 2016 Nov;50:132-140. doi: 10.1016/j.leukres.2016.10.004. Epub 2016 Oct 5. Leuk Res. 2016. PMID: 27760406 Free PMC article.
-
Phosphorylation of gH2AX as a novel prognostic biomarker for laryngoesophageal dysfunction-free survival.Oncotarget. 2016 May 31;7(22):31723-37. doi: 10.18632/oncotarget.9172. Oncotarget. 2016. PMID: 27166270 Free PMC article.
-
Antiaging agents: safe interventions to slow aging and healthy life span extension.Nat Prod Bioprospect. 2022 May 9;12(1):18. doi: 10.1007/s13659-022-00339-y. Nat Prod Bioprospect. 2022. PMID: 35534591 Free PMC article. Review.
-
Protective effect of resveratrol against caspase 3 activation in primary mouse fibroblasts.Croat Med J. 2015 Apr;56(2):78-84. doi: 10.3325/cmj.2015.56.78. Croat Med J. 2015. PMID: 25891866 Free PMC article.
-
Mesenchymal Stem Cells Support Survival and Proliferation of Primary Human Acute Myeloid Leukemia Cells through Heterogeneous Molecular Mechanisms.Front Immunol. 2017 Feb 9;8:106. doi: 10.3389/fimmu.2017.00106. eCollection 2017. Front Immunol. 2017. PMID: 28232835 Free PMC article.
References
-
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. - PubMed
-
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. - PubMed
-
- Mathon NF, Lloyd AC. Cell senescence and cancer. Nat Rev Cancer. 2001;1:203–213. - PubMed
-
- Sherr CJ, DePinho RA. Cellular senescence: Mitotic clock or culture shock? Cell. 2000;102:407–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

