Age-related changes in astrocytic and ependymal cells of the subventricular zone
- PMID: 24677590
- PMCID: PMC4322944
- DOI: 10.1002/glia.22642
Age-related changes in astrocytic and ependymal cells of the subventricular zone
Abstract
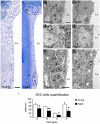
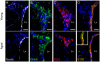
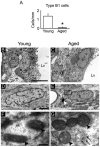
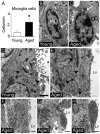
Neurogenesis persists in the adult subventricular zone (SVZ) of the mammalian brain. During aging, the SVZ neurogenic capacity undergoes a progressive decline, which is attributed to a decrease in the population of neural stem cells (NSCs). However, the behavior of the NSCs that remain in the aged brain is not fully understood. Here we performed a comparative ultrastructural study of the SVZ niche of 2-month-old and 24-month-old male C57BL/6 mice, focusing on the NSC population. Using thymidine-labeling, we showed that residual NSCs in the aged SVZ divide less frequently than those in young mice. We also provided evidence that ependymal cells are not newly generated during senescence, as others studies suggest. Remarkably, both astrocytes and ependymal cells accumulated a high number of intermediate filaments and dense bodies during aging, resembling reactive cells. A better understanding of the changes occurring in the neurogenic niche during aging will allow us to develop new strategies for fighting neurological disorders linked to senescence.
Figures


Similar articles
-
The aging neurogenic subventricular zone.Aging Cell. 2006 Apr;5(2):139-52. doi: 10.1111/j.1474-9726.2006.00197.x. Aging Cell. 2006. PMID: 16626393
-
Spatiotemporal changes to the subventricular zone stem cell pool through aging.J Neurosci. 2012 May 16;32(20):6947-56. doi: 10.1523/JNEUROSCI.5987-11.2012. J Neurosci. 2012. PMID: 22593063 Free PMC article.
-
Subventricular zone-mediated ependyma repair in the adult mammalian brain.J Neurosci. 2008 Apr 2;28(14):3804-13. doi: 10.1523/JNEUROSCI.0224-08.2008. J Neurosci. 2008. PMID: 18385338 Free PMC article.
-
Hypothalamic subependymal niche: a novel site of the adult neurogenesis.Cell Mol Neurobiol. 2014 Jul;34(5):631-42. doi: 10.1007/s10571-014-0058-5. Epub 2014 Apr 18. Cell Mol Neurobiol. 2014. PMID: 24744125 Free PMC article. Review.
-
The role of lipids in ependymal development and the modulation of adult neural stem cell function during aging and disease.Semin Cell Dev Biol. 2021 Apr;112:61-68. doi: 10.1016/j.semcdb.2020.07.018. Epub 2020 Aug 6. Semin Cell Dev Biol. 2021. PMID: 32771376 Review.
Cited by
-
Enhancing Lysosomal Activation Restores Neural Stem Cell Function During Aging.J Exp Neurosci. 2018 Aug 23;12:1179069518795874. doi: 10.1177/1179069518795874. eCollection 2018. J Exp Neurosci. 2018. PMID: 30158826 Free PMC article.
-
Pathophysiology of Lipid Droplets in Neuroglia.Antioxidants (Basel). 2021 Dec 23;11(1):22. doi: 10.3390/antiox11010022. Antioxidants (Basel). 2021. PMID: 35052526 Free PMC article. Review.
-
Vascular Senescence: A Potential Bridge Between Physiological Aging and Neurogenic Decline.Front Neurosci. 2021 Apr 20;15:666881. doi: 10.3389/fnins.2021.666881. eCollection 2021. Front Neurosci. 2021. PMID: 33958987 Free PMC article. Review.
-
Dynamic 11C-PiB PET Shows Cerebrospinal Fluid Flow Alterations in Alzheimer Disease and Multiple Sclerosis.J Nucl Med. 2019 Oct;60(10):1452-1460. doi: 10.2967/jnumed.118.223834. Epub 2019 Mar 8. J Nucl Med. 2019. PMID: 30850505 Free PMC article.
-
Optimal Extracellular Matrix Niches for Neurogenesis: Identifying Glycosaminoglycan Chain Composition in the Subventricular Neurogenic Zone.Front Neuroanat. 2021 Oct 4;15:764458. doi: 10.3389/fnana.2021.764458. eCollection 2021. Front Neuroanat. 2021. PMID: 34671246 Free PMC article. Review.
References
-
- Achanta P, Capilla-Gonzalez V, Purger D, Reyes J, Sailor K, Song H, Garcia-Verdugo JM, Gonzalez-Perez O, Ford E, Quinones-Hinojosa A. Subventricular zone localized irradiation affects the generation of proliferating neural precursor cells and the migration of neuroblasts. Stem Cells. 2012;30:2548–2560. - PMC - PubMed
-
- Batiz LF, Jimenez AJ, Guerra M, Rodriguez-Perez LM, Toledo CD, Vio K, Paez P, Perez-Figares JM, Rodriguez EM. New ependymal cells are born postnatally in two discrete regions of the mouse brain and support ven tricular enlargement in hydrocephalus. Acta Neuropathol. 2011;121:721–735. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

