Reduction of microRNA 122 expression in IFNL3 CT/TT carriers and during progression of fibrosis in patients with chronic hepatitis C
- PMID: 24672032
- PMCID: PMC4093870
- DOI: 10.1128/JVI.00016-14
Reduction of microRNA 122 expression in IFNL3 CT/TT carriers and during progression of fibrosis in patients with chronic hepatitis C
Abstract
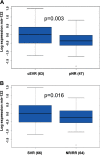
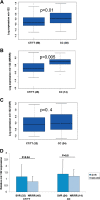
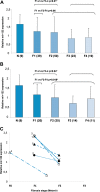
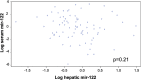
The microRNA miR-122 is highly expressed in the liver and stimulates hepatitis C virus (HCV) replication in vitro. IFNL3 (lambda-3 interferon gene) polymorphisms and the expression of miR-122 have been associated with sustained virological response (SVR) to treatment with pegylated interferon plus ribavirin in patients with chronic hepatitis C (CHC). We investigated, in vivo, the relationship between miR-122 expression, IFNL3 polymorphism, fibrosis, and response to PEG-IFN plus ribavirin. Pretreatment liver biopsy specimens and serum samples from 133 patients with CHC were included. Sixty-six patients achieved SVR, and 64 failed to respond to the treatment (43 nonresponders [NR] and 21 relapsers [RR]). All stages of fibrosis were represented, with 39, 50, 23, and 19 patients, respectively, having Metavir scores of F1, F2, F3, and F4. miR-122 expression was assessed by real-time quantitative PCR (RT-qPCR) and IFNL3 rs12979860 by direct sequencing. Hepatic miR-122 expression was higher in patients with the IFNL3 CC genotype than in those with the IFNL3 CT or TT genotype, in all patients (P = 0.025), and in NRs plus RRs (P = 0.013). Increased hepatic miR-122 was more strongly associated with complete early virological response (cEVR) (P = 0.003) than with SVR (P = 0.016). In multivariate analysis, increased hepatic miR-122 was only associated with the IFNL3 CC genotype. miR-122 was decreased in patients with advanced fibrosis (Metavir scores of F3 and F4) compared to its levels in patients with mild and moderate fibrosis (F1 and F2) (P = 0.01). Serum and hepatic expression of miR-122 were not associated. The association between miR-122 and IFNL3 was stronger than the association between miR-122 and response to treatment. miR-122 may play a role in the early viral decline that is dependent on IFNL3 and the innate immune response.
Importance: miR-122 plays a crucial role during HCV infection. Moreover, it was reported that miR-122 binding within the HCV genome stimulates its replication. Moreover, miR-122 is highly expressed within hepatocytes, where it regulates many cellular pathways. A reduction of miR-122 expression has been suggested to be associated with responsiveness to IFN-based therapy in patients with chronic hepatitis C. Several independent genome-wide association studies reported a strong association between IFNL3 polymorphism and responsiveness to IFN-based therapy. We report here a strong association between the expression of miR-122 and IFNL3 polymorphism that is independent of the response to the treatment. Our data suggest that modification of miR-122 expression may play an important role in the molecular mechanism associated with IFNL3 polymorphism. Moreover, we report a reduction of miR-122 at more advanced stages of fibrosis in patients with chronic hepatitis C.
Figures
Similar articles
-
Prevalence of IFNL3 rs4803217 single nucleotide polymorphism and clinical course of chronic hepatitis C.World J Gastroenterol. 2017 Jun 7;23(21):3815-3824. doi: 10.3748/wjg.v23.i21.3815. World J Gastroenterol. 2017. PMID: 28638221 Free PMC article.
-
Interferon-λ3 polymorphisms in pegylated-interferon-α plus ribavirin therapy for genotype-2 chronic hepatitis C.World J Gastroenterol. 2015 Apr 7;21(13):3904-11. doi: 10.3748/wjg.v21.i13.3904. World J Gastroenterol. 2015. PMID: 25852275 Free PMC article.
-
IL28B gene polymorphisms and viral kinetics in HIV/hepatitis C virus-coinfected patients treated with pegylated interferon and ribavirin.AIDS. 2011 May 15;25(8):1025-33. doi: 10.1097/QAD.0b013e3283471cae. AIDS. 2011. PMID: 21505315 Free PMC article.
-
IL28B rs12980275 variant as a predictor of sustained virologic response to pegylated-interferon and ribavirin in chronic hepatitis C patients: A systematic review and meta-analysis.Clin Res Hepatol Gastroenterol. 2015 Oct;39(5):576-83. doi: 10.1016/j.clinre.2015.01.009. Epub 2015 Mar 10. Clin Res Hepatol Gastroenterol. 2015. PMID: 25769643 Review.
-
Role of interleukin-28B polymorphism as a predictor of sustained virological response in patients with chronic hepatitis C treated with triple therapy: a systematic review and meta-analysis.Clin Drug Investig. 2013 May;33(5):325-31. doi: 10.1007/s40261-013-0074-0. Clin Drug Investig. 2013. PMID: 23532802 Review.
Cited by
-
Diagnostic and Prognostic Value of MicroRNA in Viral Diseases.Mol Diagn Ther. 2017 Feb;21(1):45-57. doi: 10.1007/s40291-016-0236-x. Mol Diagn Ther. 2017. PMID: 27682074 Review.
-
IFI35, mir-99a and HCV genotype to predict sustained virological response to pegylated-interferon plus ribavirin in chronic hepatitis C.PLoS One. 2015 Apr 6;10(4):e0121395. doi: 10.1371/journal.pone.0121395. eCollection 2015. PLoS One. 2015. PMID: 25844942 Free PMC article.
-
Parallel expression profiling of hepatic and serum microRNA-122 associated with clinical features and treatment responses in chronic hepatitis C patients.Sci Rep. 2016 Feb 22;6:21510. doi: 10.1038/srep21510. Sci Rep. 2016. PMID: 26898400 Free PMC article.
-
The Role of Interferon-λ Locus Polymorphisms in Hepatitis C and Other Infectious Diseases.J Innate Immun. 2015;7(3):231-42. doi: 10.1159/000369902. Epub 2015 Jan 23. J Innate Immun. 2015. PMID: 25634147 Free PMC article. Review.
-
MicroRNA-based diagnostic tools for advanced fibrosis and cirrhosis in patients with chronic hepatitis B and C.Sci Rep. 2016 Oct 12;6:34935. doi: 10.1038/srep34935. Sci Rep. 2016. PMID: 27731343 Free PMC article.
References
-
- Craxì A, Pawlotsky JM, Wedemeyer H, Bjoro K, Flisiak R, Forns X, Mondelli M, Peck-Radosavljevic M, Rosenberg W, Sarrazin C, Jacobson I, Dusheiko G, European Association for the Study of the Liver 2011. EASL clinical practice guidelines: management of hepatitis C virus infection. J. Hepatol. 55:245–264. 10.1016/j.jhep.2011.02.023 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

