Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis
- PMID: 24671923
- PMCID: PMC10879916
- DOI: 10.1002/14651858.CD010844.pub2
Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis
Abstract
Background: Pharmacological therapy for chronic obstructive pulmonary disease (COPD) is aimed at relieving symptoms, improving quality of life and preventing or treating exacerbations.Treatment tends to begin with one inhaler, and additional therapies are introduced as necessary. For persistent or worsening symptoms, long-acting inhaled therapies taken once or twice daily are preferred over short-acting inhalers. Several Cochrane reviews have looked at the risks and benefits of specific long-acting inhaled therapies compared with placebo or other treatments. However for patients and clinicians, it is important to understand the merits of these treatments relative to each other, and whether a particular class of inhaled therapies is more beneficial than the others.
Objectives: To assess the efficacy of treatment options for patients whose chronic obstructive pulmonary disease cannot be controlled by short-acting therapies alone. The review will not look at combination therapies usually considered later in the course of the disease.As part of this network meta-analysis, we will address the following issues.1. How does long-term efficacy compare between different pharmacological treatments for COPD?2. Are there limitations in the current evidence base that may compromise the conclusions drawn by this network meta-analysis? If so, what are the implications for future research?
Search methods: We identified randomised controlled trials (RCTs) in existing Cochrane reviews by searching the Cochrane Database of Systematic Reviews (CDSR). In addition, we ran a comprehensive citation search on the Cochrane Airways Group Register of trials (CAGR) and checked manufacturer websites and reference lists of other reviews. The most recent searches were conducted in September 2013.
Selection criteria: We included parallel-group RCTs of at least 6 months' duration recruiting people with COPD. Studies were included if they compared any of the following treatments versus any other: long-acting beta2-agonists (LABAs; formoterol, indacaterol, salmeterol); long-acting muscarinic antagonists (LAMAs; aclidinium, glycopyrronium, tiotropium); inhaled corticosteroids (ICSs; budesonide, fluticasone, mometasone); combination long-acting beta2-agonist (LABA) and inhaled corticosteroid (LABA/ICS) (formoterol/budesonide, formoterol/mometasone, salmeterol/fluticasone); and placebo.
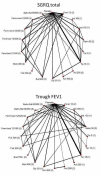
Data collection and analysis: We conducted a network meta-analysis using Markov chain Monte Carlo methods for two efficacy outcomes: St George's Respiratory Questionnaire (SGRQ) total score and trough forced expiratory volume in one second (FEV1). We modelled the relative effectiveness of any two treatments as a function of each treatment relative to the reference treatment (placebo). We assumed that treatment effects were similar within treatment classes (LAMA, LABA, ICS, LABA/ICS). We present estimates of class effects, variability between treatments within each class and individual treatment effects compared with every other.To justify the analyses, we assessed the trials for clinical and methodological transitivity across comparisons. We tested the robustness of our analyses by performing sensitivity analyses for lack of blinding and by considering six- and 12-month data separately.
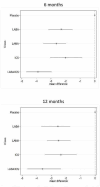
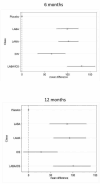
Main results: We identified 71 RCTs randomly assigning 73,062 people with COPD to 184 treatment arms of interest. Trials were similar with regards to methodology, inclusion and exclusion criteria and key baseline characteristics. Participants were more often male, aged in their mid sixties, with FEV1 predicted normal between 40% and 50% and with substantial smoking histories (40+ pack-years). The risk of bias was generally low, although missing information made it hard to judge risk of selection bias and selective outcome reporting. Fixed effects were used for SGRQ analyses, and random effects for Trough FEV1 analyses, based on model fit statistics and deviance information criteria (DIC). SGRQ SGRQ data were available in 42 studies (n = 54,613). At six months, 39 pairwise comparisons were made between 18 treatments in 25 studies (n = 27,024). Combination LABA/ICS was the highest ranked intervention, with a mean improvement over placebo of -3.89 units at six months (95% credible interval (CrI) -4.70 to -2.97) and -3.60 at 12 months (95% CrI -4.63 to -2.34). LAMAs and LABAs were ranked second and third at six months, with mean differences of -2.63 (95% CrI -3.53 to -1.97) and -2.29 (95% CrI -3.18 to -1.53), respectively. Inhaled corticosteroids were ranked fourth (MD -2.00, 95% CrI -3.06 to -0.87). Class differences between LABA, LAMA and ICS were less prominent at 12 months. Indacaterol and aclidinium were ranked somewhat higher than other members of their classes, and formoterol 12 mcg, budesonide 400 mcg and formoterol/mometasone combination were ranked lower within their classes. There was considerable overlap in credible intervals and rankings for both classes and individual treatments. Trough FEV1 Trough FEV1 data were available in 46 studies (n = 47,409). At six months, 41 pairwise comparisons were made between 20 treatments in 31 studies (n = 29,271). As for SGRQ, combination LABA/ICS was the highest ranked class, with a mean improvement over placebo of 133.3 mL at six months (95% CrI 100.6 to 164.0) and slightly less at 12 months (mean difference (MD) 100, 95% CrI 55.5 to 140.1). LAMAs (MD 103.5, 95% CrI 81.8 to 124.9) and LABAs (MD 99.4, 95% CrI 72.0 to 127.8) showed roughly equivalent results at six months, and ICSs were the fourth ranked class (MD 65.4, 95% CrI 33.1 to 96.9). As with SGRQ, initial differences between classes were not so prominent at 12 months. Indacaterol and salmeterol/fluticasone were ranked slightly better than others in their class, and formoterol 12, aclidinium, budesonide and formoterol/budesonide combination were ranked lower within their classes. All credible intervals for individual rankings were wide.
Authors' conclusions: This network meta-analysis compares four different classes of long-acting inhalers for people with COPD who need more than short-acting bronchodilators. Quality of life and lung function were improved most on combination inhalers (LABA and ICS) and least on ICS alone at 6 and at 12 months. Overall LAMA and LABA inhalers had similar effects, particularly at 12 months. The network has demonstrated the benefit of ICS when added to LABA for these outcomes in participants who largely had an FEV1 that was less than 50% predicted, but the additional expense of combination inhalers and any potential for increased adverse events (which has been established by other reviews) require consideration. Our findings are in keeping with current National Institute for Health and Care Excellence (NICE) guidelines.
Conflict of interest statement
None known.
Figures

Update of
- doi: 10.1002/14651858.CD010844
Similar articles
-
Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta-analysis.Cochrane Database Syst Rev. 2018 Dec 3;12(12):CD012620. doi: 10.1002/14651858.CD012620.pub2. Cochrane Database Syst Rev. 2018. PMID: 30521694 Free PMC article.
-
Combined aclidinium bromide and long-acting beta2-agonist for chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2018 Dec 11;12(12):CD011594. doi: 10.1002/14651858.CD011594.pub2. Cochrane Database Syst Rev. 2018. PMID: 30536566 Free PMC article.
-
Long-acting muscarinic antagonists (LAMA) added to combination long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma.Cochrane Database Syst Rev. 2016 Jan 21;2016(1):CD011721. doi: 10.1002/14651858.CD011721.pub2. Cochrane Database Syst Rev. 2016. PMID: 26798035 Free PMC article. Review.
-
Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2014 Mar 10;2014(3):CD010115. doi: 10.1002/14651858.CD010115.pub2. Cochrane Database Syst Rev. 2014. PMID: 24615270 Free PMC article. Review.
-
Efficacy of Budesonide/Glycopyrronium/Formoterol Fumarate Metered Dose Inhaler (BGF MDI) Versus Other Inhaled Corticosteroid/Long-Acting Muscarinic Antagonist/Long-Acting β2-Agonist (ICS/LAMA/LABA) Triple Combinations in COPD: A Systematic Literature Review and Network Meta-analysis.Adv Ther. 2020 Jun;37(6):2956-2975. doi: 10.1007/s12325-020-01311-3. Epub 2020 Apr 25. Adv Ther. 2020. PMID: 32335859 Free PMC article.
Cited by
-
Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: making progress towards personalised management.Lancet. 2015 May 2;385(9979):1789-1798. doi: 10.1016/S0140-6736(15)60693-6. Lancet. 2015. PMID: 25943943 Free PMC article. Review.
-
Use and outcomes associated with long-acting bronchodilators among patients hospitalized for chronic obstructive pulmonary disease.Ann Am Thorac Soc. 2014 Oct;11(8):1186-94. doi: 10.1513/AnnalsATS.201407-311OC. Ann Am Thorac Soc. 2014. PMID: 25167078 Free PMC article.
-
Agonist binding to β-adrenergic receptors on human airway epithelial cells inhibits migration and wound repair.Am J Physiol Cell Physiol. 2015 Dec 15;309(12):C847-55. doi: 10.1152/ajpcell.00159.2015. Epub 2015 Oct 21. Am J Physiol Cell Physiol. 2015. PMID: 26491049 Free PMC article.
-
Understanding how long-acting β2 -adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma - an update.Br J Pharmacol. 2016 Dec;173(24):3405-3430. doi: 10.1111/bph.13628. Epub 2016 Nov 9. Br J Pharmacol. 2016. PMID: 27646470 Free PMC article. Review.
-
Role of Tiotropium in Reducing Exacerbations of Chronic Obstructive Pulmonary Disease When Combined With Long-Acting β2 -Agonists and Inhaled Corticosteroids: The OUTPUL Study.J Clin Pharmacol. 2016 Nov;56(11):1423-1432. doi: 10.1002/jcph.750. J Clin Pharmacol. 2016. PMID: 27095425 Free PMC article.
References
References to studies included in this review
Abrahams 2013 {published data only}
-
- Abrahams R, Moroni‐Zentgraf P, Ramsdell J, Schmidt H, Joseph E, Karpel J. Safety and efficacy of the once‐daily anticholinergic BEA2180 compared with tiotropium in patients with COPD. Respiratory Medicine 2013;107(6):854‐62. - PubMed
-
- Abrahams R, Ramsdell J, Moroni‐Zentgraf P, Schmidt H, Joseph B, Karpel J. Comparison of BEA2180 to tiotropium and placebo via respimat in patients with chronic obstructive pulmonary disease (COPD) [Abstract]. Respirology 2012;17(Suppl 2):46 [292].
ACCLAIM 2009 {published data only}
-
- Jones P, Agusti A, Chanez P, Magnussen H, Fabbri L, Maroni J, et al. Efficacy and safety of aclidinium bromide, a novel long‐acting muscarinic antagonist, in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna. 2009:[P2022].
-
- Jones PE, Chanez P, Agusti A, Magnussen H, Fabbri L, Caracta C, et al. A phase III study evaluating aclidinium bromide, a novel long‐acting antimuscarinic, in patients with COPD: ACCLAIM/COPD I [Abstract]. Thorax 2009;64(Suppl IV):A168 [P213].
-
- Jones PW, Agusti A, Chanez P, Magnussen H, Fabbri L, Maroni J, et al. A phase III study evaluating aclidinium bromide, a novel long‐acting antimuscarinic, in patients with COPD: ACCLAIM/COPD 1 [Abstract]. American Thoracic Society International Conference; May 15‐20; San Diego. 2009:A6180 [Poster #207].
ACCLAIM II 2009 {published data only}
-
- Rennard S, Donohue J, Bateman E, Gross N, Garcia Gil E, Caracta C. ACCLaim/COPD ii: efficacy and safety of aclidinium bromide; a novel long‐acting muscarinic antagonist in COPD patients, a phase III study [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna. 2009:[E4351].
-
- Rennard S, Donohue J, Bateman E, Gross N, Garcia Gil E, Caracta C. Efficacy and safety of the novel, long‐acting antimuscarinic, aclidinium bromide, in COPD patients in a phase III study: ACCLAIM/COPD II [Abstract]. American Thoracic Society International Conference; May 15‐20; San Diego. 2009:A6178 [Poster #205].
Anzueto 2009 {published data only}
-
- Anzueto A, Ferguson G, Feldman G, Selbert A, Chinsky K, Emmett A, et al. A randomized, double‐blind trial comparing the effect of fluticasone/salmeterol 250/50 with salmeterol on COPD exacerbations [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:Poster #D40.
-
- Anzueto A, Ferguson GT, Feldman G, Chinsky K, Seibert A, Emmett A, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD 2009;6(5):320‐9. - PubMed
-
- Dalal AA, Blanchette CM, Petersen H, St. Charles M. Cost‐effectiveness of fluticasone propionate/salmeterol (250/50mcg) compared to salmeterol (50mcg) in patients with chronic obstructive pulmonary disease: economic evaluation of study SCO100250 [Abstract]. American Thoracic Society International Conference; May 15‐20; San Diego. 2009:A1502 [Poster #K54].
-
- GlaxoSmithKline. A randomized, double‐blind, parallel‐group, 52‐week study to compare the effect of fluticasone propionate/salmeterol DISKUS 250/50mcg BID with salmeterol DISKUS 50mcg BID on the annual rate of moderate/severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD). http://www.gsk‐clinicalstudyregister.com/ (accessed 3 March 2014).
ATTAIN 2011 {published data only}
-
- Agusti A, Jones PW, Bateman E, Singh D, Lamarca R, Miquel G, et al. Improvement in symptoms and rescue medication use with aclidinium bromide in patients with chronic obstructive pulmonary disease: results from ATTAIN [Abstract]. European Respiratory Journal 2011;38(55):149s [P874].
-
- Bateman ED, Singh D, Jones PW, Agusti A, Lamarca R, Miquel G, et al. The ATTAIN study: safety and tolerability of aclidinium bromide in chronic obstructive pulmonary disease [Abstract]. European Respiratory Journal 2011;38(55):730s [P4005].
-
- Jones P, Agusti A, Bateman E, Singh D, Lamarca R, Miquel G, et al. Aclidinium bromide in patients with chronic obstructive pulmonary disease (COPD): reduction in exacerbations as defined by health‐care utilization and the EXACT diary card [Abstract]. Chest 2011;140(4):529A.
-
- Jones P, Agusti A, Bateman E, Singh D, Lamarca R, Miquel G, et al. Aclidinium bromide in patients with chronic obstructive pulmonary disease: improvement in symptoms and health status in the ATTAIN study [Abstract]. Chest 2011;140(4):547A.
-
- Jones P, Bateman E, Singh D, Agusti A, Lamarca R, Miquel G, et al. Attain: efficacy and safety of twice‐daily aclidinium bromide in chronic obstructive pulmonary disease (COPD) [Abstract]. Chest 2011;140(4):975A.
Bateman 2010a {published data only}
-
- Bateman ED, Tashkin D, Siafakas N, Dahl R, Towse L, Massey D, et al. A one‐year trial of tiotropium Respimat plus usual therapy in COPD patients. Respiratory Medicine 2010;104(10):1460‐72. - PubMed
Bateman 2010b {published data only}
Bateman 2013 [SHINE] {published data only}
-
- Barnes N, Bateman E, Gallagher N, Green Y, Horton R, Henley M, et al. QVA149 once daily provides superior bronchodilation versus indacaterol, glycopyrronium, tiotropium and placebo: the SHINE study [Abstract]. Thorax 2012;67(Suppl 2):A147 [P192].
-
- Bateman E, Ferguson GT, Barnes N, Gallagher N, Green Y, Horton R, et al. Benefits of dual bronchodilation with QVA149 once daily versus placebo, indacaterol, NVA237 and tiotropium in patients with COPD: the SHINE study [Abstract]. European Respiratory Journal 2012;40(Suppl 56):509s [2810].
-
- Bateman E, Hashimoto S, Gallagher N, Green Y, Horton R, Henley M, et al. Glycopyrronium once daily provides rapid and sustained bronchodilation and is well tolerated in patients with COPD: the SHINE study [Abstract]. Thorax 2012;67(Suppl 2):A148 [P193].
-
- Bateman ED, Welte T, Hashimoto S, Gallagher N, Green Y, Horton R, et al. Dual bronchodilation with once‐daily QVA149 provides superior bronchodilation compared to its mono‐components and tiotropium in all subgroups of patients with COPD: the SHINE study. American Journal of Respiratory and Critical Care Medicine 2013;187:A4263.
Bourbeau 1998 {published data only}
Brusasco 2003 {published data only}
-
- Bateman ED, Hodder R, Miravitlles M, Lee A, Towse L, Serby C. A comparative trial of tiotropium, salmeterol and placebo: health‐related quality of life. European Respiratory Journal 2001;18(Suppl 33):26s.
-
- Bateman ED, Jenkins C, Korducki L, Keston S. Tiotropium (TIO) improves health care resource utilization (HRU) and patient disability in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A111.
-
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders following treatment with tiotropium and salmeterol in patients with COPD [abstract]. American Thoracic Society 99th International Conference; May 16‐21; Seattle. 2003.
-
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders over 12 hours following treatment with tiotropium or salmeterol in patients with COPD [Abstract]. Proceedings of the American Thoracic Society; May 19‐24; San Diego. 2006:A110 [Poster J10].
Burge 2000 [ISOLDE] {published data only}
-
- Bale G, Martinez‐Camblor P, Burge PS, Soriano JB. Long‐term mortality follow‐up of the ISOLDE participants: causes of death during 13 years after trial completion. Respiratory Medicine 2008;102(10):1468‐72. - PubMed
-
- Bale GA, Burge PS, Burge C, Moore VC. The Isolde study 11‐13 years on: what were the causes of death? [Abstract]. American Thoracic Society International Conference; May 18‐23; San Francisco. 2007:Poster #H42.
-
- Briggs AH, Lozano‐Ortega G, Spencer S, Bale G, Spencer MD, Burge PS. Estimating the cost‐effectiveness of fluticasone propionate for treating chronic obstructive pulmonary disease in the presence of missing data. Value in Health 2006;9(4):227‐35. - PubMed
Calverley 2003 [TRISTAN] {published data only}
-
- Calverley P, Pauwels R, Vestbo J. Erratum. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361(9369):1660. - PubMed
-
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361(9356):449‐56. - PubMed
-
- Calverley PMA, Pauwels R, Vestbo J, Jones P, Pride NB, Gulsvik A, et al. Safety of salmeterol/fluticasone propionate combination in the treatment of chronic obstructive pulmonary disease (COPD) for one year [abstract]. European Respiratory Journal 2002;20(Suppl 38):242s [P1572].
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Clinical improvements with salmeterol/fluticasone propionate combination in differing severities of COPD. American Thoracic Society 99th International Conference; May 16‐21; Seattle. 2003:A035 Poster D50.
Calverley 2003a {published data only}
-
- AstraZeneca SD. A placebo‐controlled 12‐month efficacy study of the fixed combination budesonide/formoterol compared to budesonide and formoterol as monotherapies in patients with chronic obstructive pulmonary disease (COPD). http://www.astrazenecaclinicaltrials.com/ (accessed 6 March 2014).
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference; May 21‐26; Orlando. 2004:C22 [Poster 505].
-
- Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H, et al. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(6):912‐9. - PubMed
-
- Calverley PM, Szafranski W, Calverley PMA, Andersson A. Budesonide/formoterol is a well‐tolerated long term maintenance therapy for COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1917.
-
- Calverley PMA. Effect of Budesonide/Formoterol on severe exacerbations and lung function in moderate to severe COPD. Thorax 2002;57(Suppl III):iii44.
Calverley 2003b {published data only}
-
- Calverley P, Pauwels R, Nieminem M, Stryszak P, Staudinger H, Lee T. Once daily mometasone furoate dry powder inhaler preserves lung function, reduces symptoms and delays exacerbations in patients with COPD previously maintained on ICS [Abstract]. European Respiratory Journal 2003;22(Suppl 45):Abstract No: [155].
Calverley 2007 [TORCH] {published data only}
-
- Allegra L. TORCH study: an invitation to clinical considerations. Giornale Italiano delle Malattie del Torace 2007;61(1):15‐23.
-
- Briggs AH, Glick HA, Lozano‐Ortega G, Spencer M, Calverley PM, Jones PW, et al. Is treatment with ICS and LABA cost‐effective for COPD? Multinational economic analysis of the TORCH study. European Respiratory Journal 2010;35(3):532‐9. - PubMed
-
- Briggs AH, Lozano‐Ortega G, Spencer S, Bale G, Spencer MD, Burge PS. Estimating the cost‐effectiveness of fluticasone propionate for treating chronic obstructive pulmonary disease in the presence of missing data. Value in Health 2006;9(4):227‐35. [] - PubMed
-
- Calverley P, Celli B, Anderson J, Ferguson G, Jenkins C, Jones P, et al. Salmeterol/fluticasone propionate combination (SFC) improves survival in COPD over three years: on‐treatment analysis from the TORCH study [Abstract]. American Thoracic Society International Conference; May 15‐20; San Diego. 2009:A6191[Poster #219].
-
- Calverley P, Celli B, Anderson JA, Ferguson GT, Jenkins C, Jones PW, et al. The TOwards a Revolution in COPD Health (TORCH) study: salmeterol/fluticasone propionate improves survival in COPD over three years [Abstract]. Respirology 2006;11(Suppll 5):A149 [PS‐3‐8].
Calverley 2008 {published data only}
-
- Nelson H, Karpel JP, Staudinger H, Busse WW. Mometasone furoate dry powder inhaler (MF‐DPI) improves FEV1, symptoms, and quality of life and reduces exacerbations in patients with COPD [Abstract]. Chest 2004;126(4 Suppl):709S‐c, 710.
Calverley 2010 {published data only}
-
- Calverley PMA, Kuna P, Monsó E, Costantini M, Petruzzelli S, Sergio F, et al. Beclomethasone/formoterol in the management of COPD: a randomised controlled trial. Respiratory Medicine 2010;104(12):1858‐68. - PubMed
Campbell 2005 {published data only}
-
- AstraZeneca. A 6‐month study to compare the efficacy and safety of 9microg formoterol (Oxis) Turbuhaler b.i.d. with placebo b.i.d. in patients with chronic obstructive pulmonary disease (COPD). Furthermore to compare formoterol 4.5microg as needed with terbutaline 0.5 mg as needed. SD‐037‐0709. http://www.astrazenecaclinicaltrials.com/ (accessed 12 December 2012).
-
- Bogdan M, Eliraz A, McKinnon C, Nihlen U, Radeczky E, Soliman S, et al. Formoterol turbuhaler is an effective maintenance and maintenance plus reliever therapy in patients with chronic obstructive pulmonary disease (COPD) irrespective of the level of lung function impairment and reversibility [abstract]. European Respiratory Society 12th Annual Congress; Sep 14‐18; Stockholm. 2002:abstract nr: P1578.
-
- Campbell M, Eliraz A, Johansson G, Tornling G, Nihlén U, Bengtsson T, et al. Formoterol for maintenance and as‐needed treatment of chronic obstructive pulmonary disease. Respiratory Medicine 2005;99(12):1511‐20. - PubMed
-
- Campbell M, Rabe KF, Andersson A. Formoterol turbuhaler for maintenance and as‐needed use is effective and well tolerated in patients with COPD [abstract]. American Thoracic Society 99th International Conference; May 16‐21; Seattle. 2003:B024 Poster 417.
-
- Eliraz A, Bengtsson T, Bogdan M, Coenen PDM, Johanson G, Osmanilev D, et al. Formoterol turbuhaler is effective and safe as maintenance or maintenance plus reliever therapy in patients with chronic obstructive pulmonary disease (COPD) [abstract]. European Respiratory Society 12th Annual Congress; Sep 14‐18; Stockholm. 2002:abstract nr: P1580.
Casaburi 2002 {published data only}
-
- Adams SG, Anzueto A, Briggs DD, Menjoge SS, Kesten S. Tiotropium in COPD patients not previously receiving maintenance respiratory medications. Respiratory Medicine 2006;100(9):1495‐503. - PubMed
-
- Anzueto A, Kesten S. Effects of tiotropium in COPD patients only treated with PRN albuterol [Abstract]. American Thoracic Society 100th International Conference; May 21‐26; Orlando. 2004:C47 Poster F81.
-
- Anzueto A, Tashkin D, Menjoge S, Kesten S. One‐year analysis of longitudinal changes in spirometry in patients with COPD receiving tiotropium. Pulmonary Pharmacology & Therapeutics 2005;18(2):75‐81. - PubMed
-
- Casaburi R, Mahler DA, Jones PW, Wanner A, San PG, ZuWallack RL, et al. A long‐term evaluation of once‐daily inhaled tiotropium in chronic obstructive pulmonary disease. European Respiratory Journal 2002;19(2):217‐24. - PubMed
-
- Friedman M, Bell T, Menjoge SS, Anton S, Koch P. Cost consequences of tiotropium plus existing therapy versus existing therapy alone following one year of treatment in patients with COPD. European Respiratory Journal 2001;18(Suppl 33):5s.
Chan 2007 {published data only}
Cooper 2010 {published data only}
-
- Cooper CB, Celli B, Wise RA, Jardim JR, Legg D, Guo J, et al. 'Treadmill endurance during 2 years' treatment with tiotropium in patients with COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A4586.
-
- Cooper CB, Celli BR, Wise R, Brito Jardim JR, Guo J, et al. Relationship between quality of life and exercise endurance in a long‐term study of tiotropium in COPD patients [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A1534.
Dahl 2010 {published data only}
-
- Buhl R, Pieters W, Jack D, Owen R, Kramer B, Higgins M. Indacaterol once‐daily improves dyspnoea and BODE index in COPD patients: a 52‐week study [Abstract]. European Respiratory Society Annual Congress; Sep 12‐15; Vienna. 2009:[P2026].
-
- Buhl R, Pieters W, Jack D, Owen R, Kramer B, Higgins M. Indacaterol once‐daily reduces COPD exacerbations over 52 weeks of treatment [Abstract]. American Thoracic Society International Conference; May 15‐20; San Diego. 2009:A6185 [Poster #212].
-
- Chung F, Kornmann O, Jack D, Owen R, Kramer B, Higgins M. Safety and tolerability of indacaterol over 52 weeks of treatment in COPD [Abstract]. European Respiratory Society 19th Annual Congress; Sep 12‐15; Vienna. 2009:[E4359].
-
- Chung KF, Kornmann O, Jack D, Owen R, Kramer B, Higgins M, et al. Safety and tolerability of indacaterol over 52 weeks of treatment in COPD [Abstract]. European Respiratory Society 19th Annual Congress; Sep 12‐15; Vienna. 2009:A4546 [Poster #J56].
-
- Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, et al. Efficacy of a new once‐daily long‐acting inhaled beta2‐agonist indacaterol versus twice‐daily formoterol in COPD. Thorax 2010;65(6):473‐9. - PubMed
Dal Negro 2003 {published data only}
-
- Dal Negro R, Micheletto C, Trevison F, Tognells S, Pomari C, Spencer S, et al. Salmeterol & fluticasone 50 µg/250 µg bid vs salmeterol 50 µg bid and vs placebo in the long‐term treatment of COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A228.
-
- Dal Negro RW, Pomari C, Tognella S, Micheletto C, Dal Negro R. Salmeterol & fluticasone 50 microg/250 microg bid in combination provides a better long‐term control than salmeterol 50 microg bid alone and placebo in COPD patients already treated with theophylline. Pulmonary Pharmacology and Therapeutics 2003;16(4):241‐6. - PubMed
Doherty 2012 {published data only}
-
- Doherty D, Tashkin D, Kerwin E, Eduardo Matiz‐Bueno C, Shekar T, Banerjee S, et al. Efficacy and safety of mometasone furoate/formoterol in subjects with moderate to very severe chronic obstructive pulmonary disease: results from two phase three 26‐week trials [Abstract]. Chest 2011;140(4):535A.
-
- Doherty DE, Kerwin E, Tashkin DP, Matiz‐Bueno CE, Shekar T, Banerjee S, et al. Combined mometasone furoate and formoterol in patients with moderate to very severe chronic obstructive pulmonary disease (COPD): phase 3 efficacy and safety study [Abstract]. Journal of Allergy and Clinical Immunology 2012;129(2 Suppl):AB75 [283].
-
- Doherty DE, Tashkin DP, Kerwin E, Knorr BA, Shekar T, Banerjee S, et al. Effects of mometasone furoate/formoterol fumarate fixed‐dose combination formulation on chronic obstructive pulmonary disease (COPD): results from a 52‐week Phase III trial in subjects with moderate‐to‐very severe COPD. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:57‐71. - PMC - PubMed
-
- Kerwin E, Tashkin D, Matiz‐Bueno CE, Doherty D, Shekar T, Banerjee S, et al. Quality of life following 26 weeks of mometasone furoate/formoterol therapy: results from two phase three trials in subjects with moderate to very severe chronic obstructive pulmonary disease [Abstract]. Chest 2011;140(4):558A.
-
- Matiz‐Bueno CE, Doherty D, Kerwin E, Tashkin D, Shekar T, Banerjee S, et al. The long‐term safety characteristics of mometasone furoate/formoterol for the treatment of moderate to very severe chronic obstructive pulmonary disease: pooled findings from two 1‐year multicenter clinical trials [Abstract]. Chest 2011;140(4):548A.
Donohue 2010 [INHANCE] {published data only}
-
- Barnes PJ, Pocock SJ, Magnussen H, Iqbal A, Kramer B, Higgins M, et al. Indacaterol dose selection: utilising an adaptive seamless design in a clinical study in COPD [Abstract]. European Respiratory Society 19th Annual Congress; Sep 12‐15; Vienna. 2009:[E4358].
-
- Barnes PJ, Pocock SJ, Magnussen H, Iqbal A, Kramer B, Higgins M, et al. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pulmonary Pharmacology and Therapeutics 2010;23(3):165‐71. - PubMed
-
- Barnes PJ, Pocock SJ, Magnussen H, Iqbal A, Lawrence D, Kramer B, et al. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design [Abstract]. American Thoracic Society International conference, May 15‐20, San Diego. 2009:A4543 [Poster #J53]. - PubMed
-
- Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B. Long‐term safety and efficacy of indacaterol, a long‐acting beta2‐agonist, in subjects with COPD: a randomized, placebo‐controlled study. Chest 2011;140(1):68‐75. - PubMed
-
- Donohue JF, Fogarty C, Lotvall J, Mahler DA, Worth H, Yorgancioglu A, et al. Once‐daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. American Journal of Respiratory and Critical Care Medicine 2010;182(2):155‐62. - PubMed
Dusser 2006 {published data only}
-
- Dusser D, Bravo ML, Iacono P, Lacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. European Respiratory Journal 2006;27(3):547‐55. - PubMed
-
- Dusser D, Bravo ML, Iacono P, Lacono P. Tiotropium reduces health resource utilization associated with COPD exacerbations [Abstract]. European Respiratory Journal 2004;24(Suppl 48):513s.
-
- Dusser D, Bravo ML, Ianono P, Lanono P. Tiotropium COPD exacerbations the MISTRAL study [Abstract]. European Respiratory Journal 2004;24(Suppl 48):513s.
-
- Dusser D, Viel K, Bravo ML, Iacono P. Tiotropium reduces moderate‐to‐severe exacerbations in COPD patients irrespective of concomitant use of inhaled corticosteroids (ICS) [Abstract]. American Thoracic Society International Conference; May 19‐21; San Diego. 2006:A603 [Poster H6].
-
- Dusser D, Viel K, Rouyrre N, Iancono P. Tiotropium reduces COPD exacerbations requiring treatment with systemic corticosteroids, antibiotics, or hospitalizations [Abstract]. American Thoracic Society International Conference; May 19‐21; San Diego. 2006:A603 [Poster H5].
Fang 2008 {published data only}
-
- Fang LZ, Liang X, Zhang JQ, Liu L, Fu WP, Zhao ZH, et al. Combination of inhaled salmeterol/fluticasone and tiotropium in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Chung‐Hua Chieh Ho Ho Hu Hsi Tsa Chih Chinese Journal of Tuberculosis & Respiratory Diseases 2008;31(11):811‐4. - PubMed
Ferguson 2008 {published data only}
-
- Dalal AA, Blanchette CM, Petersen H, Manavi K, St. Charles M. Cost‐effectiveness of fluticasone propionate/salmeterol (250/50 mcg) compared to salmeterol (50 mcg) in patients with COPD: economic evaluation of a randomized, double‐blind, parallel‐group, multicenter trial (Study SCO40043) [Abstract]. Chest 2008;134(4):106003s.
-
- Ferguson G, Anzueto A, Fei R, Emmett A, Crater G, Knobil K, et al. A randomized, double‐blind trial comparing the effect of fluticasone/salmeterol 250/50 to salmeterol on COPD exacerbations in patients with COPD. Chest 2007;132(4):530b‐531.
-
- Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 microg) or salmeterol (50 microg) on COPD exacerbations. Respiratory Medicine 2008;102(8):1099‐108. - PubMed
-
- SCO40043 G. A randomized, double‐blind, parallel‐group, 52‐week study to compare the effect of fluticasone propionate/salmeterol DISKUS ® 250/50mcg bid with salmeterol DISKUS ® 50mcg bid on the annual rate of moderate/severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD). http://www.gsk‐clinicalstudyregister.com/study/SCO40043#ps (accessed 3 March 2014). []
FLTA3025 {published data only}
-
- Eden HM, Price MJ, Jenkins R, Darken P, Horstman D. Fluticasone propionate improves quality of life in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A643.
-
- Rennard S, Bailey W, Tashkin D, Abrahams R, Horstman D, Ho S, et al. Improvements in airflow & dyspnea in COPD patients following BID treatment with fluticasone propionate (FP) 250mcg & 500mcg for 24 weeks via the diskus(r) inhaler [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A279.
Gelb 2012 {published data only}
-
- Gelb A, Tashkin D, Make B, Zhong X, Garcia Gil E, Caracta C. Long‐term safety of twice‐daily aclidinium bromide in COPD patients: a one‐year, double‐blind study [Abstract]. European Respiratory Society 22nd Annual Congress; Sep 1‐5; Vienna. 2012; Vol. 40, issue Suppl 56:376s [P2118].
-
- Gelb AF, Make BJ, Tashkin DP, Zhong X, Garcia GE, Caracta C. Long‐term efficacy and safety of twice‐daily aclidinium bromide in COPD patients: a one‐year study [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A2256.
-
- Tashkin D, Gelb A, Make B, Zhong X, Garcia Gil E, Caracta C. Long‐term efficacy of twice‐daily aclidinium bromide in COPD patients: a one‐year study [Abstract]. European Respiratory Society 22nd Annual Congress; Sep 1‐5; Vienna. 2012; Vol. 40, issue Suppl 56:528s [P2893].
GLOW1 2011 {published data only}
-
- D'Urzo A, Noord JA, Martin C, Horton R, Banerji D, Lu Y, et al. Once‐daily NVA237 improves symptoms, and reduces COPD exacerbations and associated hospitalisations: the GLOW1 trial [Abstract]. Thorax 2011;66(Suppl 4):P252.
-
- D'Urzo T, Ferguson G, Martin C, Alagappan VKT, Banerji D, Lu Y, et al. NVA237 once daily offers rapid and clinically meaningful bronchodilation in COPD patients that is maintained for 24 h: the GLOW1 trial [Abstract]. Thorax 2011;66(Suppl 4):P253.
-
- DUrzo A, Ferguson G, Kato M, Atis S, Martin C, Alagappan VKT, et al. NVA237 once daily provides rapid, clinically meaningful and sustained 24‐h bronchodilation in patients with COPD: the GLOW1 trial [Abstract]. European Respiratory Society 21st Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:147s [P866]. [GLOW1: NCT01005901]
-
- DUrzo A, Ferguson G, Martin C, Alagappan VKT, Banerji D, Lu Y, et al. NVA237 once daily offers rapid and clinically meaningful bronchodilation in COPD patients that is maintained for 24‐hours: the GLOW1 trial [Abstract]. 6th IPCRG World Conference; Apr 25‐28; Edinburgh. 2012:Abstract 133.
GLOW2 2012 {published data only}
-
- Kerwin EM, Hebert J, Pedinoff A, Gallagher N, Martin C, Banerji D. NVA237 once daily provides rapid and sustained bronchodilation in COPD patients, with efficacy similar to tiotropium: the GLOW2 trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A2920.
-
- Kerwin EM, Pedinoff A, Casale TB, Gallagher N, Martin C, Banerji D. NVA237 once daily reduces COPD exacerbations with similar rates to tiotropium: the GLOW2 trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A2255.
-
- Korenblat PE, Hebert J, Gallagher N, Martin C, Banerji D, Lu Y. NVA237 once daily improves dyspnea and health‐related quality of life in patients with COPD: the GLOW2 trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A2254.
Hanania 2003 {published data only}
-
- GlaxoSmithKline. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the DISKUS formulations of salmeterol (SAL) 50mcg BID and fluticasone propionate (FP) 500mcg BID individually and in combination as salmeterol 50mcg/fluticasone propionate 500mcg BID (SFC 50/500) compared to placebo in COPD subjects. http://www.gsk‐clinicalstudyregister.com/ (accessed 3 March 2014).
-
- Hanania NA. Inhaled salmeterol/fluticasone propionate combination in chronic obstructive pulmonary disease. American Journal of Respiratory Medicine 2002;1(4):284. - PubMed
-
- Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 microg)/salmeterol (50 microg) combined in the Diskus inhaler for the treatment of COPD. Chest 2003;124(3):834‐43. - PubMed
-
- Hanania NA, Knobil K, Watkins M, Wire P, Yates J, Darken P, et al. Salmeterol and fluticasone propionate therapy administered by a single diskus in patients with COPD. [Abstract]. 68th Annual Chest Meeting; Nov 2–7; San Diego. 2002:S129.
-
- Hanania NA, Ramsdell J, Payne K, Davis S, Horstman D, Lee B, et al. Improvements in airflow and dyspnea in COPD patients following 24 weeks treatment with salmeterol 50mcg and fluticasone propionate 250mcg alone or in combination via the diskus. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A279.
Kardos 2007 {published data only}
-
- GlaxoSmithKline. A multicentre, randomised, double‐blind, parallel group, placebo‐controlled study to compare the efficacy and safety of inhaled salmeterol/fluticasone propionate combination product 25/250 µg two puffs bd and fluticasone propionate 250µg two puffs bd alone, all administered via metered dose inhalers (MDI), in the treatment of subjects with chronic obstructive pulmonary disease (COPD) for 52 weeks. http://www.gsk‐clinicalstudyregister.com/ (accessed 3 March 2014).
-
- Kardos P, Wencker M. Combination therapy with salmeterol and fluticasone propionate (SFC) is more effective than salmeterol (SAL) alone in reducing exacerbations of COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1944.
-
- Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2007;175(2):144‐9. - PubMed
-
- Vogelmeier C, Wencker M, Glaab T, Kardos P. Combination therapy with salmeterol and fluticasone propionate (SFC) improves quality of life (QOL) more than salmeterol (SAL) alone in COPD [Abstract]. Norwegian Thoracic Society 42nd Nordic Lung Conference; June 9‐11; Trondheim. 2005; Vol. 15, issue Suppl 22.
-
- Vogelmeier CF, Wencker M, Glaab TH, Kardos P. Number needed to treat (NNT) to reduce exacerbations in severe COPD comparing salmeterol/fluticasone propionate (SFC) with salmeterol (SAL) treatment [Abstract]. American Thoracic Society International Conference; May 19‐21; San Diego. 2006:A110 [Poster J8].
Kornmann 2011 {published data only}
-
- Kornmann O, Dahl R, Centanni S, Dogra A, Owen R, Lassen C, et al. Once‐daily indacaterol versus twice‐daily salmeterol for COPD: a placebo‐controlled comparison. European Respiratory Journal 2011;37(2):273‐9. - PubMed
-
- Kornmann O, Luthra A, Owen R, Lassen C, Kramer B. Once‐daily indacaterol provides superior bronchodilation, health status and clinical outcomes compared with salmeterol in patients with chronic obstructive COPD: a 26‐week placebo‐controlled study [Abstract]. Chest 2009;136(4):152S.
Lapperre 2009 {published data only}
-
- Gosman MME, Lapperre TS, Snoeck‐Stroband JB, Jansen DF, Kerstjens HAM, Hiemstra PS, et al. Effect of 6 months therapy with inhaled fluticasone propionate (FP) with or without salmeterol (S) on bronchial inflammation in COPD [Abstract]. American Thoracic Society International Conference; May 19‐21; San Diego. 2006:A111 [Poster J15].
-
- Lapperre TS, Snoeck‐Stroband JB, Gosman MM, Jansen DF, Schadewijk A, Thiadens HA, et al. Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2009;151(8):517‐27. - PubMed
-
- Lapperre TS, Snoeck‐Stroband JB, Gosman MM, Sont JK, Jansen DF, Rabe KF, et al. Effects of 30 vs 6 months treatment with inhaled fluticasone (FP) with or without salmeterol (S) on bronchial inflammation in COPD [Abstract]. American Thoracic Society International Conference; May 18‐23; San Francisco. 2007:Poster #A39.
-
- Snoeck‐Stroband JB, Kerstjens HAM, Lapperre TS, Boezen HM, Thiadens HA, Hacken NHT, et al. Smoking, sputum cell counts, diffusion capacity and hyperinflation predict beneficial effects of long‐term inhaled corticosteroids (ICS) on FEV1‐decline in patients with mild‐to‐moderate COPD [Abstract]. European Respiratory Society 20th Annual Congress; Sep 18‐22; Barcelona. 2010:[P4015].
Laptseva 2002 {published data only}
-
- Laptseva IM, Laptseva EA, Borshchevsky VV, Gurevich G, Kalechits O. Inhaled budesonide in the management of chronic obstructive pulmonary diseases [abstract]. European Respiratory Society 12th Annual Congress; Sep 14‐18; Stockholm. 2002:abstract nr: P1584.
Mahler 2002 {published data only}
-
- Ferguson G, Funck‐Brentano C, Fischer T, Darken P, Troy S, Compton C, et al. Cardiovascular safety of salmeterol in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A228.
-
- Ferguson GT, Funck‐Brentano C, Fischer T, Darken P, Reisner C. Cardiovascular safety of salmeterol in COPD. Chest 2003;123(6):1817‐24. - PubMed
-
- GlaxoSmithKline. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the DISKUS formulations of salmeterol (SAL) 50mcg BID and fluticasone propionate (FP) 250mcg BID individually and in combination as salmeterol 50mcg/fluticasone propionate 250mcg BID (SFC 50/250) compared to placebo in COPD subjects. http://www.gsk‐clinicalstudyregister.com/ (accessed 3 March 2014).
-
- Littner M, Yates J, Fischer T, Horstman D, Wire P. Improvements in FEV1 and symptoms in poorly‐reversible COPD patients following 24 weeks of treatment with the salmeterol/fluticasone propionate combination 50/500 BID via the Diskus inhaler. European Respiratory Journal 2001;18(Suppl 33):176s.
-
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;166(8):1084‐91. - PubMed
Mahmud 2007 {published data only}
-
- Mahmud AM, Gupta DK, Khan AS, Hassan R, Hossain A, Rahman M, et al. Comparison of once daily tiotropium with twice daily salmeterol in Bangladeshi patients with moderate COPD [Abstract]. Respirology (Carlton, Vic.) 2007;12(Suppl 4):A211.
Niewoehner 2005 {published data only}
-
- Anonymous. Tiotropium reduces exacerbations in COPD. Pharmaceutical Journal 2004;272(7302):696.
-
- Kesten S, Plautz M, Piquette CA, Habib MP, Niewoehner DE. Premature discontinuation of patients: a potential bias in COPD clinical trials. European Respiratory Journal 2007;30(5):898‐906. - PubMed
-
- Niewoehner D, Gonzalez‐Rothi R, Shigeoka J, Korducki L, Cassino C, Kesten S, et al. Reduced COPD exacerbations in patients without first‐dose peak FEV increases 15% with tiotropium [Abstract]. American Thoracic Society International Conference; May 20‐25; San Diego. 2005:[B93] [Poster: 319].
-
- Niewoehner D, Rice K, Cote C, Korducki L, Cassino C, Kesten S. Characteristics of COPD and response to once‐daily tiotropium in African Americans in the VA medical system [Abstract]. American Thoracic Society International Conference; May 20‐25; San Diego. 2005:[A43] [Poster: F41].
-
- Niewoehner D, Rice K, Cote C, Paulson D, Cooper JA, Korducki L, et al. Reduced COPD exacerbations and associated health care utilization with once‐daily tiotropium (TIO) in the VA medical system [Abstract]. American Thoracic Society 100th International Conference, May 21‐26, Orlando. 2004:A84.
Ohar 2013 {published data only}
-
- Ohar JA, Crater G, Emmett A, Ferro T, Morris A, Raphiou I, et al. Effects of fluticasone propionate/salmeterol combination 250/50mcg bid (advair diskus) vs. salmeterol 50mcg bid (serevent diskus) on chronic obstructive pulmonary disease (COPD) exacerbation rate, following acute exacerbation or hospitalization. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A2439.
Ozol 2005 {published data only}
-
- Ozol D, Aysan T, Aytemur Z, Veral A, Sebik F. The effect of inhaled corticosteroids on bronchoalveolar lavage interleukin‐8 levels and cell percentage in stable chronic obstructive pulmonary disease patients. European Respiratory Journal 2001;18(Suppl 33):212s.
-
- Ozol D, Aysan T, Solak ZA, Mogulkoc N, Veral A, Sebik F. The effect of inhaled corticosteroids on bronchoalveolar lavage cells and IL‐8 levels in stable COPD patients. Respiratory Medicine 2005;99(12):1494‐500. - PubMed
Paggiaro 1998 {published data only}
-
- GlaxoSmithKline. A multi‐centre, randomised, double‐blind, parallel group study of the efficacy and safety of inhaled fluticasone propionate 1000 mcg daily with placebo in chronic obstructive pulmonary disease. http://www.gsk‐clinicalstudyregister.com/ (accessed 3 March 2014). [GSK: FLIT97]
-
- GlaxoSmithKline. A multicentre, double‐blind, placebo‐controlled, parallel group study of the efficacy and tolerability of long‐term inhaled fluticasone propionate 500 mcg twice daily via a Volumatic Spacer device in patients with non‐asthmatic chronic obstructive pulmonary disease, including an acute oral corticosteroid trial. http://www.gsk‐clinicalstudyregister.com/ (accessed 6 March 2014). [GSK: FLTB3054]
-
- Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo‐controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet 1998;351(9105):773‐80. - PubMed
-
- Paggiaro PL, Dahle R, Bakran I, Hollingworth K, Efthimiou J. A multicentre double‐blind, placebo‐controlled study investigating the effect of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. European Respiratory Journal 1997;10 Suppl 25:427S.
-
- Paggiaro PL, Dahle R, Bakran I, Hollingworth K, Efthimiou J. A multicentre, randomised, double‐blind, placebo‐controlled trial of inhaled fluticasone propionate (FP) in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A799.
Pauwels 1999 {published data only}
-
- Johnell O, Pauwels R, Löfdahl CG, Laitinen LA, Postma DS, Pride NB, et al. Bone mineral density in patients with chronic obstructive pulmonary disease treated with budesonide Turbuhaler(R). European Respiratory Journal 2002;19(6):1058‐63. - PubMed
-
- Liesker JJ, Velde V, Meysman M, Vinckek W, Wollmer P, Hansson L, et al. The effects of formoterol on exercise capacity in COPD. European Respiratory Society 9th Annual Congress; Oct 9‐13; Madrid. 1999:[P2511].
-
- Löfdahl CG, Postma DS, Laitinen LA, Ohlsson SV, Pauwels RA, Pride NB. The European Respiratory Society study on chronic obstructive pulmonary disease (EUROSCOP): recruitment methods and strategies. Respiratory Medicine 1998;92(3):467‐72. - PubMed
-
- Noppen M, Vincken W. Effects of high‐dose inhaled fluticasone propionate (FP) on cellular and solute composition in bronchial (Brl) and bronchoalveolar (BAL) lavage fluid in COPD: a placebo controlled, double‐blind study. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A803.
-
- Pauwels RA, Löfdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. Long‐term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease.[see comment]. New England Journal of Medicine 1999;340(25):1948‐53. - PubMed
Powrie 2007 {published data only}
-
- Powrie D, Wilkinson T, Donaldson G, Viel K, Jones P, Scrine K, et al. The effect of tiotropium on diary based exacerbation frequency in COPD [Abstract]. American Thoracic Society International Conference; May 19‐21; San Diego. 2006:A604 [Poster H11].
-
- Powrie DJ, Donaldson GC, Wilkinson TM, Jones P, Viel K, Kesten S, et al. Tiotropium reduces exacerbations and common colds in patients with COPD [Abstract]. American Thoracic Society International Conference; May 18‐23; San Francisco. 2007:Poster #A50.
-
- Powrie DJ, Wilkinson TMA, Donaldson GC, Jones P, Scrine K, Viel K, et al. Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. European Respiratory Journal 2007;30(3):472‐8. - PubMed
-
- Powrie j, Wilkinson M, Donaldson C, Wedzicha A. Tiotropium reduces subjectively reported sputum production in COPD. European Respiratory Journal 2006;28(Suppl 50):661s [P3839].
Renkema 1996 {published data only}
-
- Renkema TE, Kerstjens HA, Schouten JP, Vonk JM, Koëter GH, Postma DS. The importance of serum IgE for level and longitudinal change in airways hyperresponsiveness in COPD. Clinical and Experimental Allergy 1998;28(10):1210‐8. - PubMed
-
- Renkema TE, Schouten JP, Koëter GH, Postma DS. Effects of long‐term treatment with corticosteroids in COPD. Chest 1996;109(5):1156‐62. - PubMed
Rennard 2009 {published data only}
-
- Celli BR, Tashkin DP, Rennard SI, McElhattan J, Martin UJ. Bronchodilator responsiveness and onset of effect with budesonide/formoterol pMDI in COPD. Respiratory Medicine 2011;105(8):1176‐88. - PubMed
-
- Laties A, Rennard SI, Tashkin DP, Suchower LJ, Martin UJ. Effect of budesonide/formoterol pressurized metered‐dose inhaler (bud/fm pmdi) on ophthalmologic assessments in moderate to very severe chronic obstructive pulmonary disease (COPD) patients: results from a 1‐year, randomized, controlled clinical trial [Abstract]. Chest 2010;138(4):468A.
-
- Nelson HS, Tashkin DP, Rennard SI, Martin P, Goldman M, Silkoff PE. Onset of bronchodilation with budesonide and formoterol administered in one pressurized metered‐dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease [Abstract]. Chest 2008;134(4):105002s. - PubMed
-
- Rennard SI, Tashkin DP, Martin P, Goldman M, Martin UJ. Effect of budesonide/formoterol pressurized metered‐dose inhaler (pMDI) on exacerbations in patients with moderate to very severe chronic obstructive pulmonary disease (COPD) [Abstract]. American Thoracic Society International Conference; May 15‐20 San Diego. 2009:A1513 [Poster #K65].
Rossi 2002 {published data only}
-
- Amgott TR, Kristufek P, Levine B, Byrne A, Till D. Effect of inhaled formoterol and oral slow‐release theophylline on peak expiratory flow and symptoms in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A582.
-
- Kristufek P, Amgott TR, Levine B, Byrne A, Colacchio C. Comparison of the efficacy and safety of inhaled formoterol dry powder and oral slow‐release theophylline in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A489.
-
- Kristufek P, Levine B, Della Cioppa G, Byrne A, Till D. Bronchodilatory effects of formoterol in patients with chronic obstructive pulmonary disease (COPD) are not influenced by concomitant corticosteroid use. European Respiratory Journal 2001;18(Suppl 33):514s.
-
- Kristufek P, Levine B, Till D, Byrne A. Inhaled formoterol (foradil(r)) improves lung function in patients with both reversible and poorly reversible COPD [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A280.
-
- Rossi A, Kristufek P, Levine BE, Thomson MH, Till D, Kottakis J, et al. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow‐release theophylline in the treatment of COPD. Chest 2002;121(4):1058‐69. - PubMed
Schermer 2009 {published data only}
-
- Chavannes N, Schermer T, Woulters EFM, Folgering H, Akkermans R, Metsemakers J, et al. Demographic and clinical determinants of response to N‐acetylcysteine versus fluticasone in mild to moderate COPD in primary care: the COOPT study [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1356.
-
- Chavannes NH, Schermer TRJ, Wouters EFM, Weel C, Schayck CP. Treatment of COPD in general practice: the COOPT study. European Respiratory Journal 2001;18(Suppl 33):348s.
-
- Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, et al. Fluticasone and N‐acetylcysteine in primary care patients with COPD or chronic bronchitis. Respiratory Medicine 2009;103(4):542‐51. - PubMed
-
- Weel C, Schermer T. Quality of life is not an indicator of COPD severity. Huisarts en Wetenschap 2011;54(6):294‐7.
SCO100470 {published data only}
-
- GlaxoSmithKline. A multicentre, randomised, double‐blind, parallel group, 24‐week study to compare the effect of the salmeterol/fluticasone propionate combination product 50/250 mcg, with salmeterol 50 mcg both delivered twice daily via the DISKUS/ACCUHALER inhaler on lung function and dyspnoea in subjects with chronic obstructive pulmonary disease (COPD). http://www.gsk‐clinicalstudyregister.com/ (accessed 6 March 2014).
SCO30002 {published data only}
-
- GlaxoSmithKline. A multicentre, randomised, double‐blind, parallel group, placebo‐controlled study to compare the efficacy and safety of inhaled salmeterol/fluticasone propionate combination product 25/250 µg two puffs Bd and fluticasone propionate 250 µg two puffs Bd alone, all administered via metered dose inhalers (MDI), in the treatment of subjects with chronic obstructive pulmonary disease (COPD) for 52 weeks. http://www.gsk‐clinicalstudyregister.com/ (accessed 6 March 2014).
SCO40041 {published data only}
-
- ZuWallack R, Fogarty C, Giessel G, McClung, Locantore N, Raphiou I, et al. Effect of fluticasone propionate/salmeterol combination on change in bone mineral density in subjects with COPD [Abstract]. Chest 2008;134(4):104002s.
Senderovitz 1999 {published data only}
-
- Senderovitz T, Maltbaek N, Pedersen B, Norgaard M, Nielsen C, Vestbo J, et al. Prednisolone followed by inhaled budesonide vs placebo in chronic obstructive pulmonary disease. European Respiratory Journal 1997;10(Suppl 25):257S.
-
- Senderovitz T, Vestbo J, Frandsen J, Maltbaek N, Norgaard M, Nielsen C, et al. Steroid reversibility test followed by inhaled budesonide or placebo in outpatients with stable chronic obstructive pulmonary disease. The Danish Society of Respiratory Medicine. Respiratory Medicine 1999;93(10):715‐8. - PubMed
Shaker 2009 {published data only}
-
- Shaker SB, Dirksen A, Ulrik CS, Hestad M, Stavngaard T, Laursen LC, et al. The effect of inhaled corticosteroids on the development of emphysema in smokers assessed by annual computed tomography. COPD 2009;6(2):104‐11. - PubMed
-
- Shaker SB, Stavngaard T, Laursen LC, Stoel BC, Dirksen A. Rapid fall in lung density following smoking cessation in COPD. COPD 2011;8(1):2‐7. - PubMed
-
- Shaker SB, Stavngaard T, Stoel B, Dirksen A. The effect of inhaled corticosteroids on CT‐lung density in current smokers with COPD [Abstract]. American Thoracic Society International Conference; May 20‐25; San Diego. 2005:[A92] [Poster: 814].
Sharafkhaneh 2012 {published data only}
-
- Sharafkhaneh A, Southard J, Goldman M, Uryniak T, Martin UJ. Long‐term effect of budesonide/formoterol pressurized metered‐dose inhaler on exacerbations and pulmonary function in patients with chronic obstructive pulmonary disease [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A1599.
-
- Sharafkhaneh A, Southard JG, Goldman M, Uryniak T, Martin UJ. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double‐blind, randomized study. Respiratory Medicine 2012;106(2):257‐68. - PubMed
-
- Sharafkhaneh A, Uryniak T, Martin U. Long‐term effects of budesonide/formoterol pressurized metered‐dose inhaler on chronic obstructive pulmonary disease (COPD) symptoms and health status in patients with COPD [Abstract]. Chest 2011;140(4):528A.
-
- Southard JG, Sharafkhaneh A, Goldman M, Uryniak T, Martin UJ. Long‐term tolerability of budesonide/formoterol pressurized metered‐dose inhaler in patients with chronic obstructive pulmonary disease and a history of exacerbations [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A1597.
SLMF4010 {published data only}
-
- GlaxoSmithKline. Multicentre, randomised, parallel group, placebo‐controlled, double‐blind study, stratified on tobacco status at enrolment, evaluating during 6 months the efficacy of salmeterol powder for inhalation, 50 µg two times per day for the reduction of thoracic distension in subjects with chronic obstructive pulmonary disease (COPD). http://www.gsk‐clinicalstudyregister.com/ (accessed 6 March 2014).
SPARK 2013 {published data only}
-
- Decramer M, Wedzicha JA, Ficker J, Fowler Taylor A, D'Andrea P, Arrasate C, et al. Once‐daily QVA149 improves health‐related quality of life in patients with severe to very severe COPD: the SPARK study. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A4257.
-
- Decramer M, Wedzicha JA, Sandstrom T, Niewoehner D, Fowler Taylor A, D'Andrea P, et al. Safety and tolerability of QVA149, glycopyrronium and tiotropium in patients with severe to very severe COPD: the SPARK study. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A1479.
-
- Wedzicha JA, Decramer M, Ficker J, Fowler Taylor A, D'Andrea P, Arrasate C, et al. Dual bronchodilation with QVA149 reduces COPD exacerbations: the SPARK study. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A2428.
-
- Wedzicha JA, Decramer M, Ficker J, Niewoehner DE, Sandstrom T, Fowler Taylor A. Efficacy and safety of QVA149 versus glycopyrronium and tiotropium in severe to very severe COPD: the SPARK study. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A2429.
-
- Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandstrom T, Taylor AF, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double‐blind, parallel‐group study. The Lancet Respiratory Medicine 2013;1(3):199‐209. - PubMed
Szafranski 2003 {published data only}
-
- Anderson P. Budesonide/formoterol in a single inhaler (Symbicort) provides early and sustained improvement in lung function in moderate to severe COPD [Abstract]. Thorax 2002;57(Suppl III):iii43.
-
- AstraZeneca. A placebo‐controlled 12 month efficacy study of the fixed combination budesonide/formoterol compared with budesonide and formoterol as monotherapies in patients with chronic obstructive pulmonary disease (COPD). http://www.astrazenecaclinicaltrials.com/ (accessed 6 March 2014). [AstraZeneca: SD‐039‐0629]
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th international conference; May 21‐26; Orlando. 2004:C22 [Poster 505]. []
-
- Calverley P, Pauwels Dagger R, Lofdahl CG, Svensson K, Hi JT, Carlsson LG, et al. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. European Respiratory Journal 2005;26(3):406‐13. - PubMed
-
- Calverley PM, Szafranski W, Calverley PMA, Andersson A. Budesonide/formoterol is a well‐tolerated long term maintenance therapy for COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1917. []
Tashkin 2008 [SHINE] {published data only}
-
- AstraZeneca. A 6‐month double‐blind, double‐dummy, randomized, parallel group, multicenter efficacy & safety study of SYMBICORT® pMDI 2 x 160/4.5 mcg & 80/4.5 mcg bid compared to formoterol TBH, budesonide pMDI (& the combination) & placebo in COPD patients. http://www.astrazenecaclinicaltrials.com/ (accessed 6 March 2014).
-
- Bleecker ER, Meyers DA, Bailey WC, Sims AM, Bujac SR, Goldman M, et al. Effect of beta2‐adrenergic receptor gene polymorphism gly16arg on response to budesonide/formoterol pressurized metered‐dose inhaler in chronic obstructive pulmonary disease [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A4086. []
-
- Celli BR, Tashkin DP, Rennard SI, McElhattan J, Martin UJ. Bronchodilator responsiveness and onset of effect with budesonide/formoterol pMDI in COPD. Respiratory Medicine 2011;105(8):1176‐88. [] - PubMed
-
- Nelson HS, Tashkin DP, Rennard SI, Martin P, Goldman M, Silkoff PE. Onset of bronchodilation with budesonide and formoterol administered in one pressurized metered‐dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease [Abstract]. Chest 2008;134(4):105002s. [] - PubMed
Tashkin 2008a [UPLIFT] {published data only}
-
- Antoniu SA. UPLIFT study: the effects of long‐term therapy with inhaled tiotropium in chronic obstructive pulmonary disease. Expert Opinion on Pharmacotherapy 2009;10(4):719‐22. - PubMed
-
- Boehringer Ingelheim. A randomized, double‐blind, placebo‐controlled, parallel group trial assessing the rate of decline of lung function with tiotropium 18 mcg inhalation capsule once daily in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com (accessed 3 March 2014). []
-
- Braido F. UPLIFT study: the results and their implications. Multidisciplinary Respiratory Medicine 2009;4(Suppl 1):6s‐12s.
-
- Buhl R, Welte T, Vogelmeier C, Gillissen A, Voshaar T, Kogler H, et al. Early treatment of COPD with tiotropium. [German]. Pneumologie (Stuttgart, Germany) 2012;66(10):589‐95. - PubMed
-
- Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP, et al. Mortality in the 4‐year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2009;180(10):948‐55. - PubMed
Tashkin 2012 {published data only}
-
- Doherty D, Tashkin D, Kerwin E, Eduardo Matiz‐Bueno C, Shekar T, Banerjee S, et al. Efficacy and safety of mometasone furoate/formoterol in subjects with moderate to very severe chronic obstructive pulmonary disease: results from two phase three 26‐week trials [Abstract]. Chest 2011;140(4):535A.
-
- Kerwin E, Tashkin D, Matiz‐Bueno CE, Doherty D, Shekar T, Banerjee S, et al. Quality of life following 26 weeks of mometasone furoate/formoterol therapy: results from two phase three trials in subjects with moderate to very severe chronic obstructive pulmonary disease [Abstract]. Chest 2011;140(4):558A.
-
- Matiz‐Bueno CE, Doherty D, Kerwin E, Tashkin D, Shekar T, Banerjee S, et al. The long‐term safety characteristics of mometasone furoate/formoterol for the treatment of moderate to very severe chronic obstructive pulmonary disease: pooled findings from two 1‐year multicenter clinical trials [Abstract]. Chest 2011;140(4):548A.
-
- Tashkin D, Doherty D, Kerwin E, Matiz‐Bueno CE, Shekar T, Banerjee S, et al. The effect of mometasone furoate/formoterol combination therapy on chronic obstructive pulmonary disease (COPD) exacerbations: results from two phase three trials in subjects with moderate to very severe COPD [Abstract]. Chest 2011;140(4):549A.
-
- Tashkin DP, Doherty DE, Kerwin E, Matiz‐Bueno CE, Knorr B, Shekar T, et al. Efficacy and safety characteristics of mometasone furoate/formoterol fumarate fixed‐dose combination in subjects with moderate to very severe COPD: findings from pooled analysis of two randomized, 52‐week placebo‐controlled trials. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:73‐86. - PMC - PubMed
To 2011 {published data only}
-
- To Y, Nishimura M, Fukuchi Y, Kitawaki T, Okino N, Lassen C, et al. Long‐term safety and tolerability of indacaterol versus salmeterol in Japanese COPD patients: a 52‐week open‐labeled study [Abstract]. Respirology (Carlton, Vic.) 2011;16(Suppl 2):96 [240].
Tonnel 2008 [TIPHON] {published data only}
-
- Tonnei AB, Bravo ML, Brun M, Tonnel AB. Clinically significant improvements of health status of COPD patients after 9 months treatment with tiotropium bromide: the TIPHON study [Abstract]. American Thoracic Society International Conference; May 20‐25; San Diego. 2005:[B93] [Poster: 302].
-
- Tonnei AB, Perez T, Grosbois JM, Bravo ML, Brun M, Tonnel AB. Improvement in HRQoL of COPD patients after 9 months treatment with tiotropium bromide: use of a new scale for daily medical practice [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1934.
Trooster 2011 {published data only}
-
- Sciurba FC, Siafakas N, Troosters T, Klioze SS, Sutradhar S, Weisman I, et al. The efficacy and safety of tiotropium handihaler, 18 µg, once daily plus PRN salbutamol versus placebo plus PRN salbutamol in COPD subjects naive to maintenance therapy [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A1589.
Verhoeven 2002 {published data only}
-
- Verhoeven GT, Hegmans J, Hoogsteden HC, Prins JB. Inhaled fluticasone propionate (FP) reduces the number of inflammatory cells in bronchial biopsies of COPD patients with bronchial hyperresponsiveness (BHR). American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A798.
-
- Verhoeven GT, Mulder PGH, Bogaard JM, Hoogsteden HC. Inhaled fluticasone propionate (FP) has a significant effect on FEV1 of COPD patients with bronchial hyperresponsiveness (BHR). American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A799.
Vestbo 1999 {published data only}
-
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long‐term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 1999;353(9167):1819‐23. - PubMed
-
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long‐term effect of inhaled budesonide in patients with mild to moderate chronic obstructive lung disease. The Osterbro Study. Ugeskrift for Laeger 2000;162(4):493‐7. - PubMed
Vogelmeier 2008 {published data only}
-
- Arievich H, Potena A, Fonay K, Vogelmeier CF, Overend T, Smith J, et al. Formoterol given either alone or together with tiotropium reduces the rate of exacerbations in stable COPD patients [Abstract]. European Respiratory Journal 2006;28(Suppl 50):440s [P2514].
-
- Vogelmeier C, Kardos P, Harari S, Gans SJ, Stenglein S, Thirlwell J. Formoterol mono‐ and combination therapy with tiotropium in patients with COPD: a 6‐month study. Respiratory Medicine 2008;102(11):1511‐20. - PubMed
-
- Vogelmeier CF, Harari SA, Fonay K, Beier J, Overend T, Till D, et al. Formoterol and tiotropium both improve lung function in stable COPD patients with some additional benefit when given together [Abstract]. European Respiratory Journal 2006;28(Suppl 50):429s [P2506].
Vogelmeier 2011 [POET] {published data only}
-
- Beeh KM, Vogelmeier C, Rutten‐van Mölken M, Rabe KF, Glaab T, et al. Tiotropium vs salmeterol in GOLD II and maintenance‐naive COPD patients: subgroup analyses of POET‐COPD™ trial [Abstract]. European Respiratory Society 21st Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:20s [P251].
-
- Fabbri LM, Vogelmeier C, Rutten‐van Mölken MPMH, Rabe KF, Glaab T, Ruhmkorf F, et al. Baseline characteristics of patients with frequent exacerbations in the POET‐COPD trial [Abstract]. European Respiratory Society 21st Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:49s [405]. []
-
- Glaab T, Vogelmeier C, Schmidt H, Rutten‐van Mölken M, Beeh KM, Rabe K, et al. Seasonal distribution of exacerbations in the POET‐COPD study [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A3726. []
-
- Hoogendoorn M, Al MJ, Beeh KM, Bowles D, Graf von der Schulenburg JM, Lungershausen J, et al. Cost‐effectiveness of tiotropium versus salmeterol: the POET‐COPD trial. European Respiratory Journal 2013;41(3):556‐64. [] - PubMed
Wedzicha 2008 [INSPIRE] {published data only}
-
- Calverley P, Stockley R, Seemungal T, Hagan G, Wedzicha J. Adverse events and mortality in the INSPIRE study (investigating new standards for prophylaxis in reduction of exacerbations) [Abstract]. European Respiratory Journal 2007;30(Suppl 51):125s [P847]. - PubMed
-
- Calverley PM, Stockley RA, Seemungal TA, Hagan G, Willits LR, Riley JH, et al. Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest 2011;139(3):505‐12. - PubMed
-
- GlaxoSmithKline. Multicentre, randomised, double‐blind, double dummy, parallel group, 104‐week study to compare the effect of the salmeterol/fluticasone propionate combination product (SERETIDE*) 50/500mcg delivered twice daily via the DISKUS*/ACCUHALER* inhaler with tiotropium bromide 18 mcg delivered once daily via the HandiHaler inhalation device on the rate of health care utilisation exacerbations in subjects with severe chronic obstructive pulmonary disease (COPD). http://www.gsk‐clinicalstudyregister.com/ (accessed 6 March 2014).
-
- Seemungal T, Stockley R, Calverley P, Hagan G, Wedzicha J. Effect of salmeterol/fluticasone propionate versus tiotropium bromide on exacerbations: the INSPIRE study (investigating new standards for prophylaxis in reduction of exacerbations) [Abstract]. European Respiratory Journal 2007;30(Suppl 51):688s [E4055].
-
- Seemungal T, Stockley R, Calverley P, Hagan G, Wedzicha JA. Investigating new standards for prophylaxis in reduction of exacerbations—the INSPIRE study methodology. Journal of Chronic Obstructive Pulmonary Disease 2007;4(3):177‐83. - PubMed
Zheng 2006 {published data only}
-
- GlaxoSmithKline. A multi‐centre, randomised, double‐blind, parallel group study to investigate the efficacy and safety of the salmeterol/fluticasone propionate combination at a strength of 50/500 µg BD, compared with placebo via Accuhaler, added to usual chronic obstructive pulmonary disease (COPD) therapy, in subjects with COPD for 24 weeks. http://www.gsk‐clinicalstudyregister.com (accessed 6 March 2014).
-
- Zheng J, Zhong N, Yang L, Wu Y, Chen P, Wen Z, et al. The efficacy and safety of fluticasone propionate 500 mg/salmeterol 50 mg combined via diskus/accuhaler in Chinese patients with chronic obstructive pulmonary disease (COPD) [Abstract]. Chest 2006;130(4 Suppl):182s.
-
- Zheng JP, Yang L, Wu YM, Chen P, Wen ZG, Huang WJ, et al. The efficacy and safety of combination salmeterol (50 microg)/fluticasone propionate (500 microg) inhalation twice daily via accuhaler in Chinese patients with COPD. Chest 2007;132(6):1756‐63. - PubMed
-
- Zhong N, Zheng J, Yang L, Wu Y, Chen P, Wen Z, et al. The efficacy and safety of salmeterol 50 µg/fluticasone propionate 500 µg combined via accuhaler in Chinese patients with chronic obstructive pulmonary disease [Abstract]. Respirology (Carlton, Vic.) 2006;11(Suppl 5):A150 [PS‐3‐9].
Zhong 2012 {published data only}
-
- Zhong N, Zheng J, Wen F, Yang L, Chen P, Xiu Q, et al. Efficacy and safety of budesonide/formoterol via a dry powder inhaler in Chinese patients with chronic obstructive pulmonary disease. Current Medical Research and Opinion 2012;28(2):257‐65. - PubMed
-
- Zhong N, Zheng J, Wen F, Yang L, Chen P, Xiu Q, et al. Efficacy and safety of inhalation of budesonide/formoterol via turbuhaler in Chinese patients with chronic obstructive pulmonary disease [Abstract]. Chest 2011;140(4):5225A.
References to studies excluded from this review
Aalbers 2002 {published data only}
-
- Aalbers R, Ayres J, Backer V, Decramer M, Lier PA, Magyar P, et al. Formoterol in patients with chronic obstructive pulmonary disease: a randomized, controlled, 3‐month trial. European Respiratory Journal 2002;19(5):936‐43. [0903‐1936] - PubMed
-
- Sybrecht GW. Inhaled formoterol was an effective and safe treatment in COPD patients. European Respiratory Society 9th Annual Congress; Oct 9‐13; Madrid. 1999:2510.
ACCORD II 2012 {published data only}
-
- D'Urzo A, Kerwin E, Donohue J, Rennard S, Gelb A, Lakkis H, et al. Effects of twice‐daily aclidinium bromide in COPD patients: a long‐term extension of ACCORD‐COPD I [Abstract]. European Respiratory Society 22nd Annual Congress; Sep 1‐5; Vienna. 2012; Vol. 40, issue Suppl 56:528s [P2890]. [ACCORD: COPD II]
-
- D'Urzo A, Kerwin E, Rennard S, He T, Garcia Gil E, Caracta C. Improvements in lung function with twice‐daily aclidinium bromide: results of a long‐term, phase 3 trial in patients with chronic obstructive pulmonary disease [Abstract]. Chest 2012;142(4):740A. [ACCORD‐COPD: 1 SID ‐ NCT00891462]
-
- D'Urzo AD, Kerwin EM, Donohue JF, Rennard SI, Gelb AF, Lakkis H. Long‐term extension study of ACCORD COPD I: effects of two doses of twice‐daily aclidinium bromide in COPD patients [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A2913. [ACCORD: COPD II]
-
- Kerwin EM, D'Urzo AD, Gelb AF, Lakkis H, Garcia Gil E, Caracta CF, et al. Efficacy and safety of a 12‐week treatment with twice‐daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD 2012;9(2):90‐101. [ACCORD‐COPD: 1 SID ‐ NCT00891462] - PubMed
Ambrosino 2008 {published data only}
Auffarth 1991 {published data only}
Barnes 2006 {published data only}
-
- Barnes NC, Qiu Y, Pavord I, Parker D, Johnson M, Thompson M, et al. Salmeterol/fluticasone propionate (SFC): anti‐inflammatory effects in COPD [Abstract]. American Thoracic Society International Conference; May 20‐25; San Diego. 2005:B93 [Poster 320].
-
- Barnes NC, Qiu Y‐S, Pavord ID, Parker D, Davis PA, Zhu J, et al. Antiinflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. American Journal of Respiratory and Critical Care Medicine 2006;173(7):736‐43. - PubMed
-
- GlaxoSmithKline. A 13‐week, double‐blind, parallel‐group, multicentre study to compare the bronchial anti‐inflammatory activity of the combination of salmeterol/fluticasone propionate (SERETIDE™/ADVAIR™/VIANI™) 50/500 mcg twice daily compared with placebo twice daily in patients with chronic obstructive pulmonary disease. http://www.gsk‐clinicalstudyregister.com/ (accessed 10 June 2013). [GSK: SCO30005]
-
- Qiu Y, Parker D, Barnes NC, Johnson M, Pavord L, et al. The effect of salmeterol/fluticasone propionate (SFC) on eosinophils and mast cells in COPD [Abstract]. American Thoracic Society International Conference; May 20‐25; San Diego. 2005:A43 [Poster F36].
-
- Qiu YS, Davis P, Zhu J, Peachey L, Barnes NC, Pavord I, et al. Anti‐inflammatory effects of salmeterol/fluticasone propionate (SFC) on airway T‐lymphocyte populations in COPD. European Respiratory Journal 2005;26 Suppl 49:203s.
Beeh 2006 {published data only}
-
- Beeh KM, Beier J, Buhl R, Gerken FMN. Efficacy of tiotropium (Spiriva) in patients with COPD switched from previous treatment with short‐acting anticholinergics [Abstract]. American Thoracic Society International Conference; May 19‐21; San Diego. 2006:A113 [Poster J27].
-
- Beeh KM, Beier J, Buhl R, Stark‐Lorenzen P, Gerken F, Metzdorf N, et al. Efficacy of tiotropium bromide (Spiriva) in patients with chronic‐obstructive pulmonary disease (COPD) of different severities [Wirksamkeit von Tiotropiumbromid (Spiriva) bei verschiedenen Schweregraden derchronisch−obstruktiven Lungenerkrankung (COPD)]. Pneumologie 2006;60(6):341‐6. [CTG: NCT00274573] - PubMed
-
- Beeh KM, Beier J, Buhl R, Strak‐Lorenzen P, Gerken F, Metzdorf N. Efficacy of tiotropium in patients with mild to moderate COPD [Abstract]. American Thoracic Society 100th international conference; May 21‐26; Orlando. 2004:P513.
-
- Boehringer Ingelheim. Acute and long‐term effects of once daily oral inhalation of tiotropium 18 mcg dry powder inhalation capsules in a placebo controlled parallel group design study in patients with chronic obstructive pulmonary disease (COPD) of different severity. www.trials.boehringer‐ingelheim.com (accessed 6 March 2014). [trial number: 205.257]
Bogdan 2011 {published data only}
-
- Bogdan MA, Kudo T, Umemiya M. Efficacy and safety of inhaled formoterol 4.5 and 9 mcg twice daily in Japanese and European patients with COPD: results of a Phase III study [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181:A4494.
-
- Ichinose M, Aizawa H, Fukuchi Y, Mishima M, Nishimura M, Bogdan M. Patient‐reported outcomes (PROs) and reliever use in Japanese and European patients with chronic obstructive pulmonary disease receiving formoterol 4.5 and 9 microg twice daily: results of the OCEAN phase III study [Abstract]. European Respiratory Society 20th Annual Congress; Sep 18‐22; Barcelona. 2010.
Bourbeau 2007 {published data only}
Briggs 2005 {published data only}
-
- Briggs D Jr, Covelli H, Lapidus R, Bhattacharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared to salmeterol in COPD patients [Abstract]. American Thoracic Society 100th international conference; May 21‐26; Orlando. 2004:C22 Poster 511.
-
- Briggs DD Jr, Covelli H, Lapidus R, Bhattycharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared with salmeterol in patients with COPD. Pulmonary Pharmacology and Therapeutics 2005;18(6):397‐404. - PubMed
-
- Brusasco V, Menjoge SS, Kesten S. Flow and volume responders over 12 hours following treatment with tiotropium or salmeterol in patients with COPD [Abstract]. American Thoracic Society International Conference; May 19‐21; San Diego. 2006:A110 [Poster J10].
-
- Langley J, Briggs D Jr, Bhattacharya S, Kesten S, Cassino C. Spirometric outcomes of COPD patients with tiotropium compared with salmeterol according to previous maintenance long acting beta‐agonist use [Abstract]. European Respiratory Journal 2004;24(Suppl 48):289s.
Brightling 2005 {published data only}
Burl 2011 {published data only}
-
- Buhl R, Dunn LJ, Disdier C, Lassen C, Amos C, Henley M, et al. Blinded 12‐week comparison of once‐daily indacaterol and tiotropium in COPD. European Respiratory Journal 2011;38:797‐803. - PubMed
-
- Dunn LJ, Buhl R, Lassen C, Henley M, Kramer B. Blinded 12‐week comparison of once‐daily indacaterol and tiotropium in COPD [Abstract]. Chest 2010;138:719A. - PubMed
Cazzola 2007 {published data only}
-
- Cazzola M, Ando F, Santus P, Ruggeri P, Marco F, Sanduzzi A, et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe‐to‐very severe COPD. Pulmonary Pharmacology and Therapeutics 2007;20(5):556‐61. - PubMed
-
- D'Amato M, Ando F, Santus P, Ruggeri P, Marco F, Cazzola M. Clinical effects of adding fluticasone propionate/salmeterol (FSC) and tiotropium (TIO) in severe‐to‐very severe COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 218.
Choudhury 2005 {published data only}
-
- Choudhury AB, Dawson CM, Kilvington HE, Eldridge HE, James WY, Wedzicha JA, et al. Withdrawal of inhaled corticosteroids in people with chronic obstructive pulmonary disease (COPD) in primary care—a randomised controlled trial [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1328.
Covelli 2005 {published data only}
-
- Boehringer Ingelheim. Efficacy and safety (including 24‐hour holter monitoring) of tiotropium inhalation capsules in patients with chronic obstructive pulmonary disease (a 12‐week, parallel group, randomized, placebo‐controlled, double‐blind study). www.trials.boehringer‐ingelheim.com (accessed 6 March 2014). [trial number: 205.284]
-
- Covelli H, Bhattacharya S, Cassino C, Conoscenti C, Kesten S. Absence of electrocardiographic findings and improved function with once‐daily tiotropium in patients with chronic obstructive pulmonary disease. Pharmacotherapy 2005;25(12):1708‐18. [CTG: NCT00239460] - PubMed
-
- Kesten S, Cassino C, Conoscenti C. Absence of electrocardiographic findings with daily tiotropium in patients with chronic obstructive pulmonary disease [Abstract]. Chest 2004;126(4 Suppl):837S. - PubMed
Culpitt 1999 {published data only}
-
- Culpitt SV, Maziak W, Loukidis S, Nightingale JA, Matthews JL, Barnes PJ. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1999;160(5 Pt 1):1635‐9. - PubMed
Dahl 2001 {published data only}
-
- Dahl R, Greefhorst AP, Byrne AM. Onset of inhaled formoterol compared to ipratropium bromide in patients with COPD. European Respiratory Journal 2000;16(Suppl 31):5s.
-
- Dahl R, Greefhorst APM, Nowak D, Nonikov V, Byrne A, Colacchio C, et al. Comparison of the efficacy and safety of inhaled formoterol and ipratropium bromide in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A489.
-
- Dahl R, Greefhorst APM, Thomson MH, Till D. Formoterol (Foradil®) improves lung function and quality of life (QOL) parameters in patients with reversible or poorly reversible COPD. American Journal of Respiratory and Critical Care Medicine 2001;163(Suppl 5):A280.
-
- Dahl R, Greefhorst LA, Nowak D, Nonikov V, Byrne AM, Thomson MH, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;164(5):778‐84. [1073‐449X] - PubMed
-
- Dahl R, Kristufek P, Greefhorst APM, Amgott TR, Della Cioppa G, Thompson MH. The cardiac safety profile of formoterol dry powder is similar to placebo in patients with COPD. European Respiratory Journal 2000;16(Suppl 31):51s.
Dahl 2013 [BEACON] {published data only}
Dawber 2005 {published data only}
-
- Dawber F, Tandy D, Haussermann S, Betz R. Efficacy of salmeterol/fluticasone propionate 50/500mcg bd versus tiotropium on lung function and mucociliary clearance in COPD patients [Abstract]. Respirology (Carlton, Vic.) 2005;10(Suppl 3):A99.
Derenne 1995 {published data only}
-
- Derenne JP. Effects of high dose inhaled beclomethasone on the rate of decline in FEV1 in patients with chronic obstructive pulmonary disease: results of a 2 years prospective multicentre study. American Journal of Respiratory and Critical Care Medicine 1995;151(4):A463. []
Dransfield 2013 {published data only}
-
- Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, et al. Once‐daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double‐blind, parallel‐group, randomised controlled trials. The Lancet Respiratory Medicine 2013;1(3):210‐23. - PubMed
FCO30002 {published data only}
-
- GlaxoSmithKline. A multicentre, randomised, placebo‐controlled, double‐blind comparison with 3 parallel groups to investigate the efficacy and safety of inhaled glucocorticoid fluticasone (500 μg bd via Diskus™) vs. oral glucocorticoid therapy vs. placebo in subjects with chronic obstructive airway disease (COPD) under therapy with Salmeterol (50 μg bd). http://www.gsk‐clinicalstudyregister.com/ (accessed 6 March 2014). [GSK: FCO30002]
Ferreira 2001 {published data only}
-
- Ferreira IM, Hazari MS, Gutierrez C, Zamel N, Chapman KR. Exhaled nitric oxide and hydrogen peroxide in patients with chronic obstructive pulmonary disease: effects of inhaled beclomethasone. American Journal of Respiratory and Critical Care Medicine 2001;164(6):1012‐5. - PubMed
Ferreira 2003 {published data only}
-
- Ferreira IM, Sandrini A, Zamel N, Balter M, Chapman KR. Effects of inhaled fluticasone propionate (FP) on exhaled nitric oxide (ENO), functional exercise capacity and quality of life in stable patients with COPD. American Thoracic Society 99th International Conference; May 16‐21; Seattle. 2003:B024 Poster 404.
Freeman 2007 {published data only}
-
- Freeman D. A randomised, double‐blind, parallel group, 12 week study, comparing the effect of once daily tiotropium lactose capsule with placebo in patients with chronic obstructive pulmonary disease (COPD), naive to anticholinergic agents in addition to receiving their usual COPD care. www.trials.boehringer‐ingelheim.com (accessed 6 March 2014). [trial number: 205.276]
-
- Freeman D, Sarno M, White L, Lee A, Price D. Spruce: tiotropium in UK primary care [Abstract]. Thorax 2004;59(Suppl II):ii100.
-
- Price D, Sarno M, Lee A, Freeman D. SPiRiva usual carE—SPRUCE—tiotropium in a UK primary care COPD population other regular inhaled treatment [Abstract]. American Thoracic Society International Conference; May 20‐25; San Diego. 2005:[A43] [Poster: F26].
Fukuchi 2013 {published data only}
-
- Fukuchi Y, Samoro R, Fassakhov R, Taniguchi H, Ekelund J, Carlsson LG, et al. Budesonide/formoterol via Turbuhaler versus formoterol via Turbuhaler in patients with moderate to severe chronic obstructive pulmonary disease: phase III multinational study results. Respirology (Carlton, Vic.) 2013;18(5):866‐73. - PubMed
Guenette 2011 {published data only}
-
- Guenette JA, Raghavan N, Harris‐McAllister V, Preston ME, Webb KA, O'Donnell DE. Effect of adjunct fluticasone propionate on airway physiology during rest and exercise in COPD. Respiratory Medicine 2011;105(12):1836‐45. [1532‐3064: (Electronic)] - PubMed
Hanrahan 2008 {published data only}
-
- Baumgartner RA, Hanania NA, Calhoun WJ, Sahn SA, Sciarappa K, Hanrahan JP. Nebulized arformoterol in patients with COPD: a 12‐week, multicenter, randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled trial. Clinical Therapeutics 2007;29(2):261‐78. - PubMed
-
- Grogan DR, Hanrahan JP, Morganroth J, Cheng H, Baumgartner RA. Arrhythmias in COPD: Holter monitoring results from 2 arformoterol pivotal trials [Abstract]. American Thoracic Society International Conference; May 18‐23; San Francisco. 2007:Poster 413.
-
- Hanrahan JP, Grogan DR, Baumgartner RA, Wilson A, Cheng H, Zimetbaum PJ, et al. Arrhythmias in patients with chronic obstructive pulmonary disease (COPD): occurrence frequency and the effect of treatment with the inhaled long‐acting beta2‐agonists arformoterol and salmeterol. Medicine 2008;87(6):319‐28. - PubMed
-
- Hanrahan JP, Hanania NA, Calhoun WJ, Sahn SA, Sciarappa K, Baumgartner RA. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. Journal of Chronic Obstructive Pulmonary Disease 2008;5(1):25‐34. - PubMed
-
- Hanrahan JP, Kerwin E, Cheng H, Grogan DR, Baumgartner RA. Arformoterol in COPD safety results from two pooled phase 3 trials [Abstract]. European Respiratory Journal 2006;28(Suppl 50):428s [P2500].
Hattotuwa 2002 {published data only}
-
- Hattotuwa KL, Gizycki MJ, Ansari TW, Jeffery PK, Barnes NC. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;165(12):1592‐9. - PubMed
Johansson 2008 {published data only}
-
- Johansson G. A 12‐week, double‐blind, randomised, parallel‐group, multi‐centre study evaluating the efficacy of tiotropium versus placebo in patients with mild COPD according to Swedish guidelines (SPIRIMILD). www.trials.boehringer‐ingelheim.com (accessed 6 March 2014). [trial number: 205.281]
John 2005 {published data only}
-
- John M, Bosse S, Oltmanns U, Schumacher A, Witt C. Effects of inhaled HFA beclomethasone on pulmonary function and symptoms in patients with chronic obstructive pulmonary disease. Respiratory Medicine 2005;99(11):1418‐24. - PubMed
-
- John M, Bosse S, Schumacher A, Oltmanns U, Witt C. Effects of inhaled beclomethasone HFA 134 (qvar/ventoiair) on health related quality of life in patients with chronic obstructive pulmonary disease [Abstract]. American Thoracic Society International Conference; May 20‐25; San Diego. 2005:Poster A43.
Kerstjens 1992 {published data only}
-
- Kaptein AA, Brand PL, Dekker FW, Kerstjens HA, Postma DS, Sluiter HJ. Quality‐of‐life in a long‐term multicentre trial in chronic nonspecific lung disease: assessment at baseline. The Dutch CNSLD Study Group. European Respiratory Journal 1993;6(10):1479‐84. - PubMed
-
- Kerstjens HA, Overbeek SE, Schouten JP, Brand PL, Postma DS. Airways hyperresponsivenes, bronchodilator response, allergy and smoking predict improvement in FEV1 during long‐term inhaled corticosteroid treatment. Dutch CNSLD Study Group. European Respiratory Journal 1993;6(6):868‐76. - PubMed
Kerwin 2013 {published data only}
-
- Kerwin EM, Scott‐Wilson C, Sanford L, Rennard S, Agusti A, Barnes N, et al. A randomised trial of fluticasone furoate/vilanterol (50/25 mug; 100/25 mug) on lung function in COPD. Respiratory Medicine 2013;107(4):560‐9. - PubMed
Llewellyn‐Jones 1996 {published data only}
-
- Llewellyn‐Jones CG, Harris TA, Stockley RA. Effect of fluticasone propionate on sputum of patients with chronic bronchitis and emphysema. American Journal of Respiratory and Critical Care Medicine 1996;153(2):616‐21. - PubMed
Loppow 2001 {published data only}
-
- Loppow D, Schleiss MB, Kanniess F, Taube C, Jorres RA, Magnussen H. In patients with chronic bronchitis a four week trial with inhaled steroids does not attenuate airway inflammation. Respiratory Medicine 2001;95(2):115‐21. - PubMed
Lung Health Study 2000 {published data only}
-
- Anthonisen NR, Connett JC, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. American Journal of Respiratory and Critical Care Medicine 2002;166(5):675‐9. - PubMed
-
- Eichenhorn MS, Wise RA, Madhok TC, Gerald LB, Bailey WC, Tashkin DP, et al. Lack of long‐term adverse adrenal effects from inhaled triamcinolone: Lung Health Study II. Chest 2003;124(1):57‐62. - PubMed
-
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. New England Journal of Medicine 2000;343(26):1902‐9. - PubMed
-
- Scanlon PD, Connett JE, Wise RA, Tashkin DP, Madhok T, Skeans M, et al. Loss of one density with inhaled triamcinolone in Lung Health Study II. American Journal of Respiratory and Critical Care Medicine 2004;170(12):1302‐9. - PubMed
-
- Tashkin DP, Murray HE, Skeans M, Murray RP. Skin manifestations of inhaled corticosteroids in COPD patients. Chest 2004;126(4):1123‐33. - PubMed
Magnussen 2008 {published data only}
-
- Boehringer Ingelheim. A trial evaluating the efficacy and safety of inhaled tiotropium 18 µg qd in patients with COPD and a concomitant diagnosis of asthma. www.clinicaltrials.gov (accessed 6 March 2014).
-
- Magnussen H. A 12‐week randomised, double blind, placebo‐controlled, parallel group trial evaluating the efficacy and safety of inhaled tiotropium 18 μg q.d. in patients with COPD and a concomitant diagnosis of asthma. www.trials.boehringer‐ingelheim.com (accessed 6 March 2014). [trial number: 205.301]
-
- Magnussen H, Bugnas B, Noord J, Schmidt P, Gerken F, Kesten S. Improvements with tiotropium in COPD patients with concomitant asthma. Respiratory Medicine 2008;102(1):50‐6. [CTG: NCT00152984] - PubMed
-
- Magnussen H, Bugnas B, Noord J, Schmidt P, Gerken F, Kesten S, et al. Spirometric improvements with tiotropium in COPD patients with concomitant asthma [Abstract]. American Thoracic Society International Conference; May 18‐23; San Francisco. 2007:Poster #A5.
Mahler 1999 {published data only}
-
- Ferguson G, Funck‐Brentano C, Fischer T, Darken P, Troy S, Compton C, et al. Cardiovascular safety of salmeterol in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A228.
-
- Ferguson GT, Funck‐Brentano C, Fischer T, Darken P, Reisner C. Cardiovascular safety of salmeterol in COPD. Chest 2003;123(6):1817‐24. - PubMed
-
- GlaxoSmithKline. A randomized, double‐blind, double‐dummy, comparative clinical trial of 12‐week courses of salmeterol xinafoate versus ipratropium bromide versus placebo (PRN Ventolin) in subjects with chronic obstructive pulmonary disease. www.gsk‐clinicalstudyregister.com (accessed 20 December 2012). [GSK: SLGA4005]
-
- Mahler DA, Donohue JF, Barbee RA, Goldman MD, Gross NJ, Wisniewski ME, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999;115(4):957‐65. - PubMed
Mahler 2010 {published data only}
-
- Mahler DA, D'Urzo A, Peckitt C, Lassen C, Kramer B, Filcek S. Combining once‐daily bronchodilators In COPD: indacaterol plus tiotropium versus tiotropium alone. American Journal of Respiratory and Critical Care Medicine 2011;183:A1591.
-
- Novartis. A randomized, double‐blind, controlled, parallel group, 12‐week treatment study to compare the efficacy and safety of the combination of indacaterol 150 μg once daily with open label tiotropium 18 μg once daily versus open label tiotropium 18 μg once daily in patients with moderate‐to‐severe chronic obstructive pulmonary disease. www.novctrd.com (accessed 6 March 2014). [CQAB149B2341]
Mahler 2010a {published data only}
-
- Mahler DA, D'Urzo A, Peckitt C, Lassen C, Kramer B, Filcek S. Combining once‐daily bronchodilators In COPD: indacaterol plus tiotropium versus tiotropium alone. American Journal of Respiratory and Critical Care Medicine 2011;183:A1591.
-
- Novartis. A randomized, double‐blind, controlled, parallel group, 12‐week treatment study to compare the efficacy and safety of the combination of indacaterol 150 μg once daily with open label tiotropium 18 μg once daily versus open label tiotropium 18 μg once daily in patients with moderate‐to‐severe chronic obstructive pulmonary disease. www.novctrd.com (accessed 6 March 2014). [CQAB149B2351]
Martinez 2013 {published data only}
-
- Martinez FJ, Boscia J, Feldman G, Scott‐Wilson C, Kilbride S, Fabbri L, et al. Fluticasone furoate/vilanterol (100/25; 200/25 mug) improves lung function in COPD: a randomised trial. Respiratory Medicine 2013;107(4):550‐9. - PubMed
Mirici 2001 {published data only}
-
- Mirici A, Bektas Y, Ozbakis G, Erman Z. Effect of inhaled corticosteroids on respiratory function tests and airway inflammation in stable chronic obstructive pulmonary disease. Clinical Drug Investigation 2001;21(12):835‐42.
Moita 2008 {published data only}
-
- Boehringer Ingelheim. Spiriva® assessment of FEV1—(SAFE‐Portugal). The effect of inhaled tiotropium bromide (18 mcg once daily) on the change in FEV1 during treatment in patients with COPD. A three‐month parallel group, double‐blind, randomised, placebo‐controlled study. www.trials.boehringer‐ingelheim.com (accessed 6 March 2014). [trial number: 205.282]
-
- Moita J, Barbara C, Cardoso J, Costa R, Sousa M, Ruiz J, et al. Tiotropium improves FEV1 in patients with COPD irrespective of smoking status. Pulmonary Pharmacology and Therapeutics 2008;21(1):146‐51. [CTG: NCT00239408] - PubMed
-
- Moita J, Costa R, Barbara C, Cardosa J, Rulz J, Sousa M. The effect of tiotropium bromide in a stable cohort of COPD patients in Portugal [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1955.
NCT00144326 {published data only}
-
- García Río, F. A randomised, double‐blind, placebo‐controlled, 12 week trial to evaluate the effect of tiotropium inhalation capsules on the magnitude of exercise, measured using an accelerometer, in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com (accessed 15 October 2007). [CTG: NCT00144326; trial number: 205.269]
Nelson 2007 {published data only}
-
- Gross NJ, Lapidus R, Dunn LJ, Lynn LD, Denis‐Mize K, Rinehart M. Nebulized formoterol is an effective bronchodilator and improves the quality of life for COPD patients [Abstract]. American Thoracic Society International Conference; May 18‐23; San Francisco. 2007:Poster A10.
-
- Gross NJ, Nelson HS, Lapidus RJ, Dunn L, Lynn L, Rinehart M, et al. Efficacy and safety of formoterol fumarate delivered by nebulization to COPD patients. Respiratory Medicine 2008;102(2):189‐97. - PubMed
-
- Nelson HS, Gross NJ, Levine B, Kerwin EM, Rinehart M, Denis‐Mize K, et al. Cardiac safety profile of nebulized formoterol in adults with COPD: a 12‐week, multicenter, randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled trial. Clinical Therapeutics 2007;29(10):2167‐78. - PubMed
-
- Nelson HS, Gross NJ, Rinehart M, Denis‐Mize K. Cardiovascular safety of nebulized formoterol in COPD patients: a double‐blind, placebo‐controlled study. Chest 2007;132(4):529b‐530.
-
- Nelson HS, ZuWallack R, Levine B, Kerwin EM, Denis‐Mize K, Rinehart M. Safety profile of formoterol fumarate delivered by nebulization to COPD patients [Abstract]. American Thoracic Society International Conference; May 18‐23; San Francisco. 2007:Poster A11.
Nishimura 1999 {published data only}
-
- Nishimura K, Koyama H, Ikeda A, Tsukino M, Hajiro T, Mishima M, et al. The effect of high‐dose inhaled beclomethasone dipropionate in patients with stable COPD. Chest 1999;115(1):31‐7. - PubMed
O'Donnell 2006 {published data only}
-
- Celli B, Emmett A, Crater G, Kalberg C. Salmeterol/fluticasone propionate (SFC) improves the inspiratory to total lung capacity ratio (IC/TLC) and exercise endurance time in patients with COPD. European Respiratory Journal 2006;28(Suppl 50):764s.
-
- GlaxoSmithKline. A randomized, double‐blind, placebo‐controlled, parallel group clinical trial evaluating the effect of the fluticasone propionate/salmeterol combination product 250/50mcg bid via DISKUS and salmeterol 50mcg bid via DISKUS on lung hyperinflation in subjects with chronic obstructive pulmonary disease (COPD). http://www.gsk‐clinicalstudyregister.com/ (accessed 10 June 2013). [GSK: SCO40030]
-
- Make B, Emmett A, Crater G, O'Dell D, Kalberg C. Improvement in exercise endurance time (EET) with fluticasone propionate/salmeterol correlations with spirometry and plethysmography. American Thoracic Society International Conference; May 19‐21; San Diego. 2006.
-
- O'Donnell DE, Sciurba F, Celli B, Mahler DA, Webb KA, Kalberg CJ, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest 2006;130(3):647‐56. - PubMed
Rennard 2001 {published data only}
-
- Ferguson G, Funck‐Brentano C, Fischer T, Darken P, Troy S, Compton C, et al. Cardiovascular safety of salmeterol in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A228.
-
- Ferguson GT, Funck‐Brentano C, Fischer T, Darken P, Reisner C. Cardiovascular safety of salmeterol in COPD. Chest 2003;123(6):1817‐24. - PubMed
-
- GlaxoSmithKline. A randomized, double‐blind, double‐dummy, comparative clinical trial of 12‐week courses of salmeterol xinafoate versus ipratropium bromide versus placebo (PRN Ventolin®) in subjects with chronic obstructive pulmonary disease. www.gsk‐clinicalstudyregister.com (accessed 7 December 2012). [GSK: SLGA4004]
-
- Rennard SI, Anderson W, ZuWallack R, Broughton J, Bailey W, Friedman M, et al. Use of a long‐acting inhaled beta2‐adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2001;163(5):1087‐92. - PubMed
Robertson 1986 {published data only}
-
- Robertson AS, Gove RI, Wieland GA, Burge PS. A double‐blind comparison of oral prednisolone 40 mg/day with inhaled beclomethasone dipropionate 1500 ug/day in patients with adult onset chronic obstructive airways disease. European Journal of Respiratory Diseases 1986;146(Suppl):565‐9. - PubMed
Rutgers 1998 {published data only}
-
- Rutgers SR, Koeter GH, Mark TW, Postma DS. Short‐term treatment with budesonide does not improve hyperresponsiveness to adenosine 5'‐monophosphate in COPD. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Pt 1):880‐6. - PubMed
SCO40034 {published data only}
-
- GlaxoSmithKline. A multicentre, randomised, double‐blind, double dummy, parallel group 12‐week exploratory study to compare the effect of the fluticasone/salmeterol propionate combination product (SERETIDE™) 50/500mcg bd via the DISKUS™/ACCUHALER™ inhaler with tiotropium bromide 18 mcg od via the Handihaler inhalation device on efficacy and safety in patients with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files/pdf/23678.pdf (accessed 16 June 2009). [GSK: SCO40034]
Sin 2004 {published data only}
-
- Sin DD, Lacy P, York E, Man SFP. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2004;170(7):760‐5. - PubMed
Sin 2008 {published data only}
-
- Sin DD, Man SF, Marciniuk DD, Ford G, FitzGerald M, Wong E, et al. ABC (Advair, Biomarkers in COPD) Investigators. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2008;177(11):1207‐14. - PubMed
Sun 2007 {published data only}
-
- Sun LH, Tan Y, Qiao Y, Fang SR, Xie H. Evaluation of clinical effect and safety of tiotropium bromide in treating stable chronic obstructive pulmonary disease. Zhongguo Xinyao yu Linchuang Zazhi 2007;26(5):328‐31.
-
- Yan L, Lu YK. Effect of domestic tiotropium bromide inhalation in patients with COPD at stable stage. Chinese Journal of New Drugs 2010;19(2):127‐9, 138.
Tashkin 2009 {published data only}
-
- Tashkin D, Varghese S. Formoterol treatment plus tiotropium results in greater improvements in lung function compared with tiotropium administered alone in patients with COPD [Abstract]. Journal of Allergy and Clinical Immunology 2007;119(1 Suppl):S4 [13].
-
- Tashkin D, Varghese S. The therapeutic effect of treatment with formoterol plus tiotropium was greater than the effect of treatment with tiotropium alone in COPD: findings from a 12‐week, multicenter, double‐blind, placebo‐controlled trial. Chest 2007;132(4):529a.
-
- Tashkin DP. Formoterol and tiotropium reduces rescue medication use more than tiotropium alone in patients with moderate COPD: findings from a 12 week randomized placebo controlled trial [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:A647[#F5].
-
- Tashkin DP, Pearle J, Iezzoni D, Varghese ST. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. Journal of Chronic Obstructive Pulmonary Disease 2009;6(1):17‐25. - PubMed
-
- Tashkin DP, Pearle JL, Varghese S. Improvement of lung function with coadministered formoterol and tiotropium, regardless of smoking status in patients with chronic obstructive pulmonary disease [Abstract]. Chest 2008;134(4):103002s.
Thompson 1992 {published data only}
-
- Thompson AB, Mueller MB, Heires AJ, Bohling TL, Daughton D, Yancey SW, et al. Aerosolized beclomethasone in chronic bronchitis: improved pulmonary function and diminished airway inflammation. American Review of Respiratory Disease 1992;146(2):389‐95. [MEDLINE: ] - PubMed
Thompson 2002 {published data only}
-
- Thompson WH, Carvalho P, Souza JP, Charan NB. Controlled trial of inhaled fluticasone propionate in moderate to severe COPD. Lung 2002;180(4):191‐201. - PubMed
van der Valk 2002 {published data only}
-
- Valk P, Monninkhof E, Palen J, Zielhuis G, Herwaarden C, Phalen J. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. American Journal of Respiratory and Critical Care Medicine 2002;166(10):1358‐63. - PubMed
van Grunsven 2003 {published data only}
-
- Grunsven P, Schermer T, Akkermans R, Albers M, Boom G, Schayck O, et al. Short‐ and long‐term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respiratory Medicine 2003;97(12):1303‐12. - PubMed
Verkinde 2006 {published data only}
-
- Huchon G. Effect of a 12‐week treatment with inhaled tiotropium (18 mcg once daily) on lung function and static lung volumes in stable, moderate to severe COPD patients. Correlation to dyspnoea scales: a double‐blind, placebo‐controlled, randomized, parallel group study. www.trials.boehringer‐ingelheim.com (accessed 6 March 2014). [trial number: 205.215]
-
- Huchon G, Verkindre C, Bart F, Aguilaniu B, Guerin JC, Lemerre C, et al. Improvements with tiotropium on endurance measure by the shuttle walking test (SWT) and on health related quality of life (HRQoL) in COPD patients. European Respiratory Society 12th Annual Congress; Sep 14‐18; Stockholm. 2002.
-
- Verkindre C, Bart F, Aguilaniu B, Fortin F, Guerin JC, Merre C, et al. The effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary disease. Respiration 2006;73(4):420‐7. - PubMed
Voshaar 2008 {published data only}
-
- Boehringer Ingelheim. A randomised, double‐blind, double‐dummy, placebo‐ and active‐controlled, parallel group efficacy and safety comparison of 12‐week treatment of two doses [5 mcg (2 actuations of 2.5 mcg) and 10 mcg (2 actuations of 5 mcg)] of tiotropium inhalation solution delivered by the Respimat inhaler, placebo and ipratropium bromide inhalation aerosol (MDI) in patients with chronic obstructive pulmonary disease (COPD). www.trials.boehringer‐ingelheim.com (accessed 6 March 2014). [trial number: 205.251]
-
- Boehringer Ingelheim. A randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled, parallel group efficacy and safety comparison of 12‐week treatment of two doses (5 Mcg and 10 Mcg) of tiotropium inhalation solution delivered by the Respimat inhaler, placebo and ipratropium bromide inhalation aerosol (MDI) in patients with chronic obstructive pulmonary disease (COPD). www.clinicaltrials.gov (accessed 6 March 2014). [trial number: 205.252]
-
- Voshaar T, Lapidus R, Maleki‐Yazdi R, Timmer W, Rubin E, Lowe L, et al. A randomised study of tiotropium Respimat soft mist inhaler versus ipratropium PMDI in chronic obstructive pulmonary disease [Abstract]. Thorax 2006;61(Suppl 2):ii40 [S112].
-
- Voshaar T, Lapidus R, Maleki‐Yazdi R, Timmer W, Rubin E, Lowe L, et al. A randomized study of tiotropium Respimat Soft Mist inhaler vs. ipratropium pMDI in COPD. Respiratory Medicine 2008;102(1):32‐41. [CTG: NCT00239473; CTG: NCT00240435] - PubMed
Wadbo 2002 {published data only}
-
- Wadbo M, Lofdahl CG, Larsson K, Skoogh BE, Tornling G, Arwestrom E, et al. Effects of formoterol and ipratropium bromide in COPD: a 3‐month placebo‐controlled study. European Respiratory Journal 2002;20(5):1138‐46. [0903‐1936] - PubMed
Watkins 2002 {published data only}
-
- Watkins M, Wire P, Yates J, Fischer T, Chang C, Horstman D, et al. Sustained FEV increases in COPD patients induced by salmeterol 50 mcg twice daily via the diskus inhaler. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A228. - PubMed
Weiner 1995 {published data only}
-
- Weiner P, Weiner M, Azgad Y, Zamir D. Inhaled budesonide therapy for patients with stable COPD. Chest 1995;108(6):1568‐71. - PubMed
Weiner 1999 {published data only}
-
- Weiner P, Weiner M, Rabner M, Waizman J, Magadle R, Zamir D. The response to inhaled and oral steroids in patients with stable chronic obstructive pulmonary disease. Journal of Internal Medicine 1999;245(1):83‐9. - PubMed
Weir 1990 {published data only}
-
- Weir DC, Gove RI, Robertson AS, Burge PS. Response to corticosteroids in chronic airflow obstruction: relationship to emphysema and airways collapse. European Respiratory Journal 1991;4(10):1185‐90. - PubMed
Weir 1999 {published data only}
-
- Weir DC, Bale GA, Bright P, Sherwood Burge P. A double‐blind placebo‐controlled study of the effect of inhaled beclomethasone dipropionate for 2 years in patients with nonasthmatic chronic obstructive pulmonary disease. Clinical and Experimental Allergy Supplement 1999;29(2):125‐8. - PubMed
Welte 2009 {published data only}
-
- Welte T, Hartman L, Polanowski T, Hernandez P. Budesonide/formoterol added to tiotropium is well tolerated and reduces risk of severe exacerbations in COPD patients [Abstract]. American Thoracic Society International Conference; May 15‐20 San Diego. 2009:A6188 [Poster #215].
-
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Addition of budesonide/formoterol to tiotropium reduces the number of exacerbation days compared with tiotropium alone. Chest 2009;136(4):26S‐f.
-
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Budesonide/formoterol added to tiotropium provides rapid improvements in lung function and ability to undertake morning activities. Chest 2009;136(4):24S‐g.
-
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2009;180(8):741‐50. - PubMed
-
- Welte T, Miravitlles M, Hernandez P, Hartman L, Polanowski T, Kessler R. Budesonide/formoterol added to tiotropium improves lung function, health status, symptoms & morning activities in COPD patients [Abstract]. European Respiratory Society 19th Annual Congress; Sep 12‐15; Vienna. 2009:[P2005].
Wempe 1992 {published data only}
Wouters 2005 {published data only}
-
- Rizzato G. COPD: immediate and sustained deterioration after withdrawal of fluticasone in patients under therapy with salmeterol + fluticasone. [Italian]. Internista 2005;13(4):225‐30.
-
- Wouters EF, Postma DS, Fokkens B, Hop WC, Prins J, Kuipers AF, et al. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax 2005;60(6):480‐7. - PMC - PubMed
Yang 2012 {published data only}
-
- Yang W, Liu J, Gao B, Cheng K. Effect of fluticasone propionate/salmeterol on exercise endurance in moderate‐severe COPD [Abstract]. European Respiratory Society 22nd Annual Congress; Sep 1‐5; Vienna. 2012; Vol. 40:624s [P3459].
Yildiz 2004 {published data only}
-
- Yildiz F, Basyigit I, Yildirim E, Boyaci H, Ilgazli A. Does addition of inhaled steroids to combined bronchodilator therapy affect health status in patients with COPD?. Respirology 2004;9(3):352‐5. - PubMed
References to ongoing studies
INSTEAD {unpublished data only}
-
- Comparison of Indacaterol 150 μg Once Daily (o.d.) With Salmeterol/Fluticasone Propionate 50 μg/500 μg Twice Daily (b.i.d.) (INSTEAD). clinicaltrials.gov (accessed 5 December 2013).
Vestbo 2013 {published data only}
-
- Vestbo J, Anderson J, Brook RD, Calverley PMA, Celli BR, Crim C, et al. The study to understand mortality and morbidity in COPD (SUMMIT) study protocol. European Respiratory Journal 2013;41(5):1017‐22. - PubMed
Additional references
Appleton 2006
ATS/ERS 2004
-
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. European Respiratory Journal 2004;23(6):932‐46. - PubMed
Barr 2005
Beeh 2010
-
- Beeh KM, Beier J. The short, the long, and the "ultra‐long": why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Advances in Therapy 2010;27(3):150‐9. - PubMed
Berger 2008
-
- Berger WE, Nadel JA. Efficacy and safety of formoterol for the treatment of chronic obstructive pulmonary disease. Respiratory Medicine 2008;102(2):173‐88. - PubMed
Cazzola 2008
-
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. European Respiratory Journal 2008;31(2):416‐69. - PubMed
Chong 2012
Chuck 2008
Cipriani 2013
-
- Cipriani A, Higgins JPT, Geddes JR, Salanti GS. Conceptual and technical challenges in network meta‐analysis. Annals of Internal Medicine 2013;159(2):130‐7. - PubMed
Cooper 2009
-
- Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between‐study heterogeneity and inconsistency in mixed treatment comparisons: application to stroke prevention treatments in individuals with non‐rheumatic atrial fibrillation. Statistics in Medicine 2009;28:1861‐81. - PubMed
Cope 2012
Cope 2013
Decramer 2013
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. - PubMed
Dias 2013
Dias 2013a
Dong 2013
-
- Dong Y, Lin H, Shau W, Wu Y, Chang C, Lai M. Comparative safety of inhaled medications inpatients with chronic obstructive pulmonary disease:systematic review and mixed treatment comparison meta‐analysis of randomised controlled trials. Thorax 2013;68:48‐56. - PubMed
Earnshaw 2007
-
- Earnshaw SR, Wilson MR, Dalal AA, Chambers MG, Jhingran P, Stanford R, et al. Cost‐effectiveness of fluticasone propionate/salmeterol (500/50 microg) in the treatment of COPD. Respiratory Medicine 2009;103(1):12‐21. - PubMed
Effing 2007
Fattore 2005
-
- Fattore G, Torbica A, Mangone M. Cost‐analysis of four treatment strategies in the management of moderate‐to‐severe COPD: an application of non‐parametric bootstrap [Italian]. Pharmacoeconomics Italian Research Articles 2005;7(2):135‐43.
Geake 2012
GOLD
-
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013. http://www.goldcopd.com (accessed 3 October 2013).
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org 2011.
Higgins 2012
Hutchinson 2010
-
- Hutchinson A, Brand C, Irving L, Roberts C, Thompson P, Campbell D. Acute care costs of patients admitted for management of chronic obstructive pulmonary disease exacerbations: contribution of disease severity, infection and chronic heart failure. Internal Medicine Journal 2010;40(5):364‐71. - PubMed
Karner 2011
-
- Karner C, Cates CJ. Combination inhaled steroid and long‐acting beta2‐agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 3. [Airways code: TIO2‐COP; DOI: 10.1002/14651858.CD008532] - DOI - PMC - PubMed
Karner 2011a
Karner 2012
-
- Karner C, Cates CJ. Long‐acting beta2‐agonist in addition to tiotropium versus either tiotropium or long‐acting beta2‐agonist alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 4. [Airways code: TIO3‐COP; DOI: 10.1002/14651858.CD008989] - DOI - PMC - PubMed
Karner 2012a
Kew 2013
Kew 2014
Lacasse 2006
Loke 2011
-
- Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta‐analysis of randomised controlled trials and observational studies. Thorax 2011;66(8):699‐708. - PubMed
Lu 2006
-
- Lu G, Ades A. Assessing evidence consistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101:447‐59.
Moen 2010
-
- Moen MD. Indacaterol: in chronic obstructive pulmonary disease. Drugs 2010;70(17):2269‐80. - PubMed
Nannini 2012
-
- Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006829; CCB3‐COP] - DOI
Nannini 2013
-
- Nannini LJ, Poole P, Milan SJ, Holmes R, Normansell R. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 11. [Airways code: CCB‐COP; DOI: 10.1002/14651858.CD003794] - DOI - PMC - PubMed
Nannini 2013a
-
- Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 8. [Airways code: CCB2‐COP; DOI: 10.1002/14651858.CD006826] - DOI - PMC - PubMed
NICE 2011
-
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease, costing report, implementing NICE guidance. http://guidance.nice.org.uk/CG101/CostingReport/pdf/English (accessed 3 October 2013).
Puhan 2009
Puhan 2011
Rodrigo 2008
-
- Rodrigo GJ, Nannini LJ, Rodríguez‐Roisin R. Safety of long‐acting β‐agonists in stable COPD: a systematic review. Chest 2008;133(5):1079‐87. - PubMed
Rodrigo 2009
-
- Rodrigo GJ, Castro‐Rodriguez JA, Plaza V. Safety and efficacy of combined long‐acting beta‐agonists and inhaled corticosteroids vs long‐acting beta‐agonists monotherapy for stable COPD: a systematic review. Chest 2009;136(4):1029‐38. - PubMed
Sestini 2009
Singh 2010
Spencer 2011
-
- Spencer S, Karner C, Cates CJ, Evans DJ. Inhaled corticosteroids versus long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 12. [Airways code: IST‐COP; DOI: 10.1002/14651858.CD007033] - DOI
Spiegelhalter 2002
-
- Spiegelhalter DJ, Best NG, Carlin BP, Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society. Series B (Statistical Methodology) 2002;64(4):583‐639.
Tashkin 2008
-
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. New England Journal of Medicine 2008;359(15):1543‐54. - PubMed
van der Meer 2001
Welsh 2013
WHO
-
- World Health Organization. Chronic respiratory diseases. http://www.who.int/respiratory/en/ (accessed 3 October 2013).
Wise 2013
-
- Wise RA, Anzueto A, Cotton D, Dahl R, Devins T Disse B, et al. Tiotropium Respimat inhaler and the risk of death in COPD. New England Journal of Medicine Vol. 369, issue 16:1491‐501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

