Sphingosine-1-phosphate receptor 1 reporter mice reveal receptor activation sites in vivo
- PMID: 24667638
- PMCID: PMC4001537
- DOI: 10.1172/JCI71194
Sphingosine-1-phosphate receptor 1 reporter mice reveal receptor activation sites in vivo
Abstract
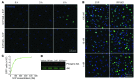
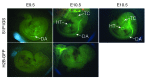
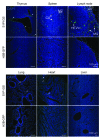
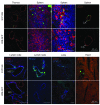
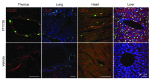
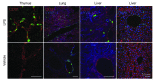
Activation of the GPCR sphingosine-1-phosphate receptor 1 (S1P1) by sphingosine-1-phosphate (S1P) regulates key physiological processes. S1P1 activation also has been implicated in pathologic processes, including autoimmunity and inflammation; however, the in vivo sites of S1P1 activation under normal and disease conditions are unclear. Here, we describe the development of a mouse model that allows in vivo evaluation of S1P1 activation. These mice, known as S1P1 GFP signaling mice, produce a S1P1 fusion protein containing a transcription factor linked by a protease cleavage site at the C terminus as well as a β-arrestin/protease fusion protein. Activated S1P1 recruits the β-arrestin/protease, resulting in the release of the transcription factor, which stimulates the expression of a GFP reporter gene. Under normal conditions, S1P1 was activated in endothelial cells of lymphoid tissues and in cells in the marginal zone of the spleen, while administration of an S1P1 agonist promoted S1P1 activation in endothelial cells and hepatocytes. In S1P1 GFP signaling mice, LPS-mediated systemic inflammation activated S1P1 in endothelial cells and hepatocytes via hematopoietically derived S1P. These data demonstrate that S1P1 GFP signaling mice can be used to evaluate S1P1 activation and S1P1-active compounds in vivo. Furthermore, this strategy could be potentially applied to any GPCR to identify sites of receptor activation during normal physiology and disease.
Figures
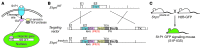

Similar articles
-
HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation.Sci Signal. 2015 Aug 11;8(389):ra79. doi: 10.1126/scisignal.aaa2581. Sci Signal. 2015. PMID: 26268607 Free PMC article.
-
Sphingosine-1-phosphate-induced Flk-1 transactivation stimulates mouse embryonic stem cell proliferation through S1P1/S1P3-dependent β-arrestin/c-Src pathways.Stem Cell Res. 2014 Jan;12(1):69-85. doi: 10.1016/j.scr.2013.08.013. Epub 2013 Sep 7. Stem Cell Res. 2014. PMID: 24145189
-
Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells.Circ Res. 2008 Nov 7;103(10):1164-72. doi: 10.1161/01.RES.0000338501.84810.51. Epub 2008 Oct 10. Circ Res. 2008. PMID: 18849324 Free PMC article.
-
Relevance and potential of sphingosine-1-phosphate in vascular inflammatory disease.Biol Chem. 2008 Nov;389(11):1381-90. doi: 10.1515/BC.2008.165. Biol Chem. 2008. PMID: 18925828 Review.
-
Vascular sphingosine-1-phosphate S1P1 and S1P3 receptors.Drug News Perspect. 2004 Jul-Aug;17(6):365-82. doi: 10.1358/dnp.2004.17.6.829028. Drug News Perspect. 2004. PMID: 15334188 Review.
Cited by
-
S1PR1 regulates the quiescence of lymphatic vessels by inhibiting laminar shear stress-dependent VEGF-C signaling.JCI Insight. 2020 Jul 23;5(14):e137652. doi: 10.1172/jci.insight.137652. JCI Insight. 2020. PMID: 32544090 Free PMC article.
-
Aging Suppresses Sphingosine-1-Phosphate Chaperone ApoM in Circulation Resulting in Maladaptive Organ Repair.Dev Cell. 2020 Jun 22;53(6):677-690.e4. doi: 10.1016/j.devcel.2020.05.024. Epub 2020 Jun 15. Dev Cell. 2020. PMID: 32544390 Free PMC article.
-
Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy.J Clin Invest. 2015 Apr;125(4):1379-87. doi: 10.1172/JCI76369. Epub 2015 Apr 1. J Clin Invest. 2015. PMID: 25831442 Free PMC article. Review.
-
Targeting cancer metabolism by simultaneously disrupting parallel nutrient access pathways.J Clin Invest. 2016 Nov 1;126(11):4088-4102. doi: 10.1172/JCI87148. Epub 2016 Sep 26. J Clin Invest. 2016. PMID: 27669461 Free PMC article.
-
Phosphorylation of a constrained azacyclic FTY720 analog enhances anti-leukemic activity without inducing S1P receptor activation.Leukemia. 2017 Mar;31(3):669-677. doi: 10.1038/leu.2016.244. Epub 2016 Aug 30. Leukemia. 2017. PMID: 27573555 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

