The N-terminal domain of the androgen receptor drives its nuclear localization in castration-resistant prostate cancer cells
- PMID: 24662325
- PMCID: PMC4127361
- DOI: 10.1016/j.jsbmb.2014.03.004
The N-terminal domain of the androgen receptor drives its nuclear localization in castration-resistant prostate cancer cells
Abstract
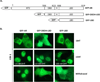
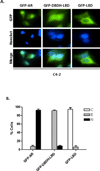
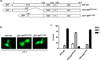
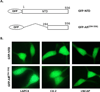
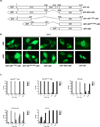
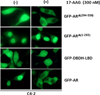
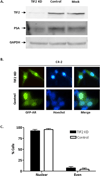
Androgen-independent nuclear localization is required for androgen receptor (AR) transactivation in castration-resistant prostate cancer (CRPC) and should be a key step leading to castration resistance. However, mechanism(s) leading to androgen-independent AR nuclear localization are poorly understood. Since the N-terminal domain (NTD) of AR plays a role in transactivation under androgen-depleted conditions, we investigated the role of the NTD in AR nuclear localization in CRPC. Deletion mutagenesis was used to identify amino acid sequences in the NTD essential for its androgen-independent nuclear localization in C4-2, a widely used CRPC cell line. Deletion mutants of AR tagged with green fluorescent protein (GFP) at the 5'-end were generated and their signal distribution was investigated in C4-2 cells by fluorescent microscopy. Our results showed that the region of a.a. 294-556 was required for androgen-independent AR nuclear localization whereas a.a. 1-293 mediates Hsp90 regulation of AR nuclear localization in CRPC cells. Although the region of a.a. 294-556 does not contain a nuclear import signal, it was able to enhance DHT-induced import of the ligand binding domain (LBD). Also, transactivation of the NTD could be uncoupled from its modulation of AR nuclear localization in C4-2 cells. These observations suggest an important role of the NTD in AR intracellular trafficking and androgen-independent AR nuclear localization in CRPC cells.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Regulation and targeting of androgen receptor nuclear localization in castration-resistant prostate cancer.J Clin Invest. 2021 Feb 15;131(4):e141335. doi: 10.1172/JCI141335. J Clin Invest. 2021. PMID: 33332287 Free PMC article.
-
N-terminal domain of the androgen receptor contains a region that can promote cytoplasmic localization.J Steroid Biochem Mol Biol. 2014 Jan;139:16-24. doi: 10.1016/j.jsbmb.2013.09.013. Epub 2013 Oct 4. J Steroid Biochem Mol Biol. 2014. PMID: 24099702 Free PMC article.
-
Androgen receptor splice variants activate androgen receptor target genes and support aberrant prostate cancer cell growth independent of canonical androgen receptor nuclear localization signal.J Biol Chem. 2012 Jun 1;287(23):19736-49. doi: 10.1074/jbc.M112.352930. Epub 2012 Apr 24. J Biol Chem. 2012. PMID: 22532567 Free PMC article.
-
Androgen receptors in hormone-dependent and castration-resistant prostate cancer.Pharmacol Ther. 2013 Dec;140(3):223-38. doi: 10.1016/j.pharmthera.2013.07.003. Epub 2013 Jul 13. Pharmacol Ther. 2013. PMID: 23859952 Review.
-
Androgen receptor: what we know and what we expect in castration-resistant prostate cancer.Int Urol Nephrol. 2018 Oct;50(10):1753-1764. doi: 10.1007/s11255-018-1964-0. Epub 2018 Aug 20. Int Urol Nephrol. 2018. PMID: 30128923 Review.
Cited by
-
Development and Implementation of a High-Throughput High-Content Screening Assay to Identify Inhibitors of Androgen Receptor Nuclear Localization in Castration-Resistant Prostate Cancer Cells.Assay Drug Dev Technol. 2016 May;14(4):226-39. doi: 10.1089/adt.2016.716. Assay Drug Dev Technol. 2016. PMID: 27187604 Free PMC article.
-
The classical and updated models of androgen receptor nucleocytoplasmic trafficking.Am J Clin Exp Urol. 2021 Aug 25;9(4):287-291. eCollection 2021. Am J Clin Exp Urol. 2021. PMID: 34541027 Free PMC article. Review.
-
A new compound targets the AF-1 of androgen receptor and decreases its activity and protein levels in prostate cancer cells.Am J Cancer Res. 2020 Dec 1;10(12):4607-4623. eCollection 2020. Am J Cancer Res. 2020. PMID: 33415022 Free PMC article.
-
Androgen receptor nucleocytoplasmic trafficking - A one-way journey.Mol Cell Endocrinol. 2023 Oct 1;576:112009. doi: 10.1016/j.mce.2023.112009. Epub 2023 Jul 4. Mol Cell Endocrinol. 2023. PMID: 37414131 Free PMC article. Review.
-
Inhibition of Androgen Receptor Nuclear Localization and Castration-Resistant Prostate Tumor Growth by Pyrroloimidazole-based Small Molecules.Mol Cancer Ther. 2017 Oct;16(10):2120-2129. doi: 10.1158/1535-7163.MCT-17-0176. Epub 2017 Jun 27. Mol Cancer Ther. 2017. PMID: 28655783 Free PMC article.
References
-
- Isaacs JT, Coffey DS. Etiology and disease process of benign prostatic hyperplasia. The Prostate. Supplement. 1989;2:33. - PubMed
-
- Kozlowski JM, Ellis WJ, Grayhack JT. Advanced prostatic carcinoma. Early versus late endocrine therapy. The Urologic clinics of North America. 1991 Feb;18:15. - PubMed
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010 Sep-Oct;60:277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

