α6β2*-subtype nicotinic acetylcholine receptors are more sensitive than α4β2*-subtype receptors to regulation by chronic nicotine administration
- PMID: 24661093
- PMCID: PMC4107044
- DOI: 10.1111/jnc.12721
α6β2*-subtype nicotinic acetylcholine receptors are more sensitive than α4β2*-subtype receptors to regulation by chronic nicotine administration
Abstract
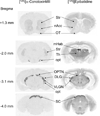
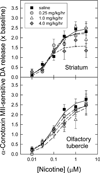
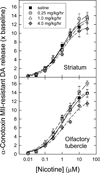
Nicotinic acetylcholine receptors (nAChR) of the α6β2* subtype (where *indicates the possible presence of additional subunits) are prominently expressed on dopaminergic neurons. Because of this, their role in tobacco use and nicotine dependence has received much attention. Previous studies have demonstrated that α6β2*-nAChR are down-regulated following chronic nicotine exposure (unlike other subtypes that have been investigated - most prominently α4β2* nAChR). This study examines, for the first time, effects across a comprehensive chronic nicotine dose range. Chronic nicotine dose-responses and quantitative ligand-binding autoradiography were used to define nicotine sensitivity of changes in α4β2*-nAChR and α6β2*-nAChR expression. α6β2*-nAChR down-regulation by chronic nicotine exposure in dopaminergic and optic-tract nuclei was ≈three-fold more sensitive than up-regulation of α4β2*-nAChR. In contrast, nAChR-mediated [(3) H]-dopamine release from dopamine-terminal region synaptosomal preparations changed only in response to chronic treatment with high nicotine doses, whereas dopaminergic parameters (transporter expression and activity, dopamine receptor expression) were largely unchanged. Functional measures in olfactory tubercle preparations were made for the first time; both nAChR expression levels and nAChR-mediated functional measures changed differently between striatum and olfactory tubercles. These results show that functional changes measured using synaptosomal [(3) H]-DA release are primarily owing to changes in nAChR, rather than in dopaminergic, function. This study examined dose-response relationships for murine α6β2*-nicotinic acetylcholine receptor (nAChR) down-regulation by chronic nicotine treatment. The ID50 value for α6β2* down-regulation (35 nM) is ≈ 3x lower than the ED50 value for α4β2* nAChR up-regulation (95 nM), both well within the range reached by human smokers. Chronic nicotine treatment altered α6β2*- and α4β2*-nAChR-mediated [(3) H]-dopamine release from striatal and olfactory tubercle synaptosomes, but dopaminergic parameters were largely unaffected. We conclude that functional changes are primarily driven by altered nAChR activity.
Keywords: dopamine receptor; dopamine transporter; dopaminergic terminal regions; nicotinic receptor; α4β2 subtype.
© 2014 International Society for Neurochemistry.
Conflict of interest statement
The authors declare that they have no conflicts of interest associated with the work described in this manuscript.
Figures



Similar articles
-
UB-165: a novel nicotinic agonist with subtype selectivity implicates the alpha4beta2* subtype in the modulation of dopamine release from rat striatal synaptosomes.J Neurosci. 2000 Apr 15;20(8):2783-91. doi: 10.1523/JNEUROSCI.20-08-02783.2000. J Neurosci. 2000. PMID: 10751429 Free PMC article.
-
Long-term nicotine treatment differentially regulates striatal alpha6alpha4beta2* and alpha6(nonalpha4)beta2* nAChR expression and function.Mol Pharmacol. 2008 Sep;74(3):844-53. doi: 10.1124/mol.108.048843. Epub 2008 Jun 26. Mol Pharmacol. 2008. PMID: 18583454 Free PMC article.
-
Selectivity of ABT-089 for alpha4beta2* and alpha6beta2* nicotinic acetylcholine receptors in brain.Biochem Pharmacol. 2009 Oct 1;78(7):795-802. doi: 10.1016/j.bcp.2009.05.022. Epub 2009 May 27. Biochem Pharmacol. 2009. PMID: 19481067 Free PMC article.
-
The contribution of agonist and antagonist activities of α4β2* nAChR ligands to smoking cessation efficacy: a quantitative analysis of literature data.Psychopharmacology (Berl). 2018 Sep;235(9):2479-2505. doi: 10.1007/s00213-018-4921-9. Epub 2018 Jul 7. Psychopharmacology (Berl). 2018. PMID: 29980822 Review.
-
The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum.Biochem Pharmacol. 2007 Oct 15;74(8):1235-46. doi: 10.1016/j.bcp.2007.07.032. Epub 2007 Jul 27. Biochem Pharmacol. 2007. PMID: 17825262 Free PMC article. Review.
Cited by
-
Chronic treatment with varenicline changes expression of four nAChR binding sites in mice.Neuropharmacology. 2015 Dec;99:142-55. doi: 10.1016/j.neuropharm.2015.07.019. Epub 2015 Jul 17. Neuropharmacology. 2015. PMID: 26192545 Free PMC article.
-
α4 nicotinic receptors on GABAergic neurons mediate a cholinergic analgesic circuit in the substantia nigra pars reticulata.Acta Pharmacol Sin. 2024 Jun;45(6):1160-1174. doi: 10.1038/s41401-024-01234-7. Epub 2024 Mar 4. Acta Pharmacol Sin. 2024. PMID: 38438581
-
Chronic Nicotine Exposure Attenuates Methamphetamine-Induced Dopaminergic Deficits.J Pharmacol Exp Ther. 2015 Dec;355(3):463-72. doi: 10.1124/jpet.114.221945. Epub 2015 Sep 21. J Pharmacol Exp Ther. 2015. PMID: 26391161 Free PMC article.
-
Sex Differences in the Nicotinic Acetylcholine Receptor System of Rodents: Impacts on Nicotine and Alcohol Reward Behaviors.Front Neurosci. 2021 Sep 21;15:745783. doi: 10.3389/fnins.2021.745783. eCollection 2021. Front Neurosci. 2021. PMID: 34621155 Free PMC article. Review.
-
Neural circuits and nicotinic acetylcholine receptors mediate the cholinergic regulation of midbrain dopaminergic neurons and nicotine dependence.Acta Pharmacol Sin. 2020 Jan;41(1):1-9. doi: 10.1038/s41401-019-0299-4. Epub 2019 Sep 25. Acta Pharmacol Sin. 2020. PMID: 31554960 Free PMC article. Review.
References
-
- Barr JE, Holmes DB, Ryan LJ, Sharpless SK. Techniques for the chronic cannulation of the jugular vein in mice. Pharmacol Biochem Behav. 1979;11:115–118. - PubMed
-
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

