Decreased O-linked GlcNAcylation protects from cytotoxicity mediated by huntingtin exon1 protein fragment
- PMID: 24648514
- PMCID: PMC4036360
- DOI: 10.1074/jbc.M114.553321
Decreased O-linked GlcNAcylation protects from cytotoxicity mediated by huntingtin exon1 protein fragment
Abstract
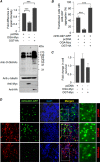
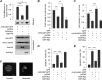
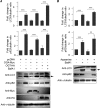
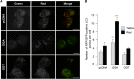
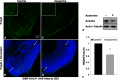
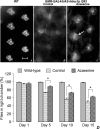
O-GlcNAcylation is an important post-translational modification of proteins and is known to regulate a number of pathways involved in cellular homeostasis. This involves dynamic and reversible modification of serine/threonine residues of different cellular proteins catalyzed by O-linked N-acetylglucosaminyltransferase and O-linked N-acetylglucosaminidase in an antagonistic manner. We report here that decreasing O-GlcNAcylation enhances the viability of neuronal cells expressing polyglutamine-expanded huntingtin exon 1 protein fragment (mHtt). We further show that O-GlcNAcylation regulates the basal autophagic process and that suppression of O-GlcNAcylation significantly increases autophagic flux by enhancing the fusion of autophagosome with lysosome. This regulation considerably reduces toxic mHtt aggregates in eye imaginal discs and partially restores rhabdomere morphology and vision in a fly model for Huntington disease. This study is significant in unraveling O-GlcNAcylation-dependent regulation of an autophagic process in mediating mHtt toxicity. Therefore, targeting the autophagic process through the suppression of O-GlcNAcylation may prove to be an important therapeutic approach in Huntington disease.
Keywords: Autophagy; Neurodegenerative Diseases; O-GlcNAcylation; Post-translational Modification; Protein Aggregation.
Figures
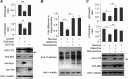


Similar articles
-
O-GlcNAcylation modulates HBV replication through regulating cellular autophagy at multiple levels.FASEB J. 2020 Nov;34(11):14473-14489. doi: 10.1096/fj.202001168RR. Epub 2020 Sep 6. FASEB J. 2020. PMID: 32892442
-
HIPK3 modulates autophagy and HTT protein levels in neuronal and mouse models of Huntington disease.Autophagy. 2018;14(1):169-170. doi: 10.1080/15548627.2017.1393130. Epub 2018 Jan 29. Autophagy. 2018. PMID: 29130397 Free PMC article.
-
Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain.Nat Commun. 2015 Apr 13;6:6768. doi: 10.1038/ncomms7768. Nat Commun. 2015. PMID: 25866135 Free PMC article.
-
Genetic manipulations of mutant huntingtin in mice: new insights into Huntington's disease pathogenesis.FEBS J. 2013 Sep;280(18):4382-94. doi: 10.1111/febs.12418. Epub 2013 Jul 31. FEBS J. 2013. PMID: 23829302 Free PMC article. Review.
-
[Huntington's disease: intracellular signaling pathways and neuronal death].J Soc Biol. 2005;199(3):247-51. doi: 10.1051/jbio:2005026. J Soc Biol. 2005. PMID: 16471265 Review. French.
Cited by
-
O-GlcNAc modification is essential for the regulation of autophagy in Drosophila melanogaster.Cell Mol Life Sci. 2015 Aug;72(16):3173-83. doi: 10.1007/s00018-015-1889-z. Epub 2015 Apr 4. Cell Mol Life Sci. 2015. PMID: 25840568 Free PMC article.
-
Modulation of O-GlcNAcylation Regulates Autophagy in Cortical Astrocytes.Oxid Med Cell Longev. 2019 Nov 13;2019:6279313. doi: 10.1155/2019/6279313. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31827688 Free PMC article.
-
In vitro selection of blackberry (Rubus fruticosus 'Tupy') plants resistant to Botrytis cinerea using gamma ray-irradiated shoot tips.Plant Biotechnol (Tokyo). 2022 Jun 25;39(2):165-171. doi: 10.5511/plantbiotechnology.22.0312b. Plant Biotechnol (Tokyo). 2022. PMID: 35937526 Free PMC article.
-
Glycogen synthase protects neurons from cytotoxicity of mutant huntingtin by enhancing the autophagy flux.Cell Death Dis. 2018 Feb 8;9(2):201. doi: 10.1038/s41419-017-0190-5. Cell Death Dis. 2018. PMID: 29422655 Free PMC article.
-
O-GlcNAc regulation of autophagy and α-synuclein homeostasis; implications for Parkinson's disease.Mol Brain. 2017 Jul 19;10(1):32. doi: 10.1186/s13041-017-0311-1. Mol Brain. 2017. PMID: 28724388 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

