Retrograde BMP signaling modulates rapid activity-dependent synaptic growth via presynaptic LIM kinase regulation of cofilin
- PMID: 24647957
- PMCID: PMC3960475
- DOI: 10.1523/JNEUROSCI.4943-13.2014
Retrograde BMP signaling modulates rapid activity-dependent synaptic growth via presynaptic LIM kinase regulation of cofilin
Abstract
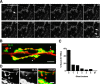
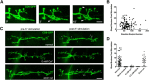
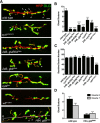
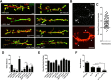
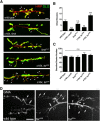
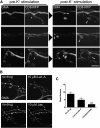
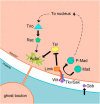
The Drosophila neuromuscular junction (NMJ) is capable of rapidly budding new presynaptic varicosities over the course of minutes in response to elevated neuronal activity. Using live imaging of synaptic growth, we characterized this dynamic process and demonstrated that rapid bouton budding requires retrograde bone morphogenic protein (BMP) signaling and local alteration in the presynaptic actin cytoskeleton. BMP acts during development to provide competence for rapid synaptic growth by regulating the levels of the Rho-type guanine nucleotide exchange factor Trio, a transcriptional output of BMP-Smad signaling. In a parallel pathway, we find that the BMP type II receptor Wit signals through the effector protein LIM domain kinase 1 (Limk) to regulate bouton budding. Limk interfaces with structural plasticity by controlling the activity of the actin depolymerizing protein Cofilin. Expression of constitutively active or inactive Cofilin in motor neurons demonstrates that increased Cofilin activity promotes rapid bouton formation in response to elevated synaptic activity. Correspondingly, the overexpression of Limk, which inhibits Cofilin, inhibits bouton budding. Live imaging of the presynaptic F-actin cytoskeleton reveals that activity-dependent bouton addition is accompanied by the formation of new F-actin puncta at sites of synaptic growth. Pharmacological disruption of actin turnover inhibits bouton budding, indicating that local changes in the actin cytoskeleton at pre-existing boutons precede new budding events. We propose that developmental BMP signaling potentiates NMJs for rapid activity-dependent structural plasticity that is achieved by muscle release of retrograde signals that regulate local presynaptic actin cytoskeletal dynamics.
Keywords: BMP; Drosophila; actin; neuromuscular junction; synapse formation; synaptic plasticity.
Figures
Similar articles
-
Retrograde BMP signaling at the synapse: a permissive signal for synapse maturation and activity-dependent plasticity.J Neurosci. 2013 Nov 6;33(45):17937-50. doi: 10.1523/JNEUROSCI.6075-11.2013. J Neurosci. 2013. PMID: 24198381 Free PMC article.
-
BMP signaling modulates the probability of neurotransmitter release and readily releasable pools in Drosophila neuromuscular junction synapses.Biochem Biophys Res Commun. 2016 Oct 21;479(3):440-446. doi: 10.1016/j.bbrc.2016.09.072. Epub 2016 Sep 23. Biochem Biophys Res Commun. 2016. PMID: 27671198
-
LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor.Neuron. 2005 Sep 1;47(5):695-708. doi: 10.1016/j.neuron.2005.08.010. Neuron. 2005. PMID: 16129399
-
LIM Kinases, Promising but Reluctant Therapeutic Targets: Chemistry and Preclinical Validation In Vivo.Cells. 2022 Jun 30;11(13):2090. doi: 10.3390/cells11132090. Cells. 2022. PMID: 35805176 Free PMC article. Review.
-
Structural Aspects of LIMK Regulation and Pharmacology.Cells. 2022 Jan 2;11(1):142. doi: 10.3390/cells11010142. Cells. 2022. PMID: 35011704 Free PMC article. Review.
Cited by
-
Role of BMP receptor traffic in synaptic growth defects in an ALS model.Mol Biol Cell. 2016 Oct 1;27(19):2898-910. doi: 10.1091/mbc.E16-07-0519. Epub 2016 Aug 17. Mol Biol Cell. 2016. PMID: 27535427 Free PMC article.
-
Neuronal LRP4 directs the development, maturation, and cytoskeletal organization of peripheral synapses.bioRxiv [Preprint]. 2023 Nov 8:2023.11.03.564481. doi: 10.1101/2023.11.03.564481. bioRxiv. 2023. Update in: Development. 2024 Jun 1;151(11):dev202517. doi: 10.1242/dev.202517 PMID: 37961323 Free PMC article. Updated. Preprint.
-
Reactive oxygen species regulate activity-dependent neuronal plasticity in Drosophila.Elife. 2018 Dec 17;7:e39393. doi: 10.7554/eLife.39393. Elife. 2018. PMID: 30540251 Free PMC article.
-
The Nature of Diamino Linker and Halogen Bonding Define Selectivity of Pyrrolopyrimidine-Based LIMK1 Inhibitors.Front Chem. 2021 Dec 13;9:781213. doi: 10.3389/fchem.2021.781213. eCollection 2021. Front Chem. 2021. PMID: 34966720 Free PMC article.
-
Drosophila Synaptotagmin 7 negatively regulates synaptic vesicle release and replenishment in a dosage-dependent manner.Elife. 2020 Apr 28;9:e55443. doi: 10.7554/eLife.55443. Elife. 2020. PMID: 32343229 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
