Neural map formation in the mouse olfactory system
- PMID: 24638094
- PMCID: PMC4111858
- DOI: 10.1007/s00018-014-1597-0
Neural map formation in the mouse olfactory system
Abstract
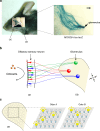
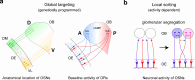
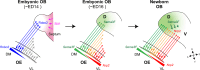
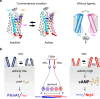
In the mouse olfactory system, odorants are detected by ~1,000 different odorant receptors (ORs) produced by olfactory sensory neurons (OSNs). Each OSN expresses only one functional OR species, which is referred to as the "one neuron-one receptor" rule. Furthermore, OSN axons bearing the same OR converge to a specific projection site in the olfactory bulb (OB) forming a glomerular structure, i.e., the "one glomerulus-one receptor" rule. Based on these basic rules, binding signals of odorants detected by OSNs are converted to topographic information of activated glomeruli in the OB. During development, the glomerular map is formed by the combination of two genetically programmed processes: one is OR-independent projection along the dorsal-ventral axis, and the other is OR-dependent projection along the anterior-posterior axis. The map is further refined in an activity-dependent manner during the neonatal period. Here, we summarize recent progress of neural map formation in the mouse olfactory system.
Figures

Similar articles
-
Developmental regulation of neural map formation in the mouse olfactory system.Dev Neurobiol. 2015 Jun;75(6):594-607. doi: 10.1002/dneu.22268. Epub 2015 Feb 18. Dev Neurobiol. 2015. PMID: 25649346 Review.
-
Odorant receptor gene choice and axonal projection in the mouse olfactory system.Results Probl Cell Differ. 2009;47:57-75. doi: 10.1007/400_2008_3. Results Probl Cell Differ. 2009. PMID: 19083127 Review.
-
How is the olfactory map formed and interpreted in the mammalian brain?Annu Rev Neurosci. 2011;34:467-99. doi: 10.1146/annurev-neuro-112210-112917. Annu Rev Neurosci. 2011. PMID: 21469960 Review.
-
Hierarchical regulation of odorant receptor gene choice and subsequent axonal projection of olfactory sensory neurons in zebrafish.J Neurosci. 2007 Feb 14;27(7):1606-15. doi: 10.1523/JNEUROSCI.4218-06.2007. J Neurosci. 2007. PMID: 17301169 Free PMC article.
-
Developmental regulation of olfactory circuit formation in mice.Dev Growth Differ. 2020 May;62(4):199-213. doi: 10.1111/dgd.12657. Epub 2020 Feb 28. Dev Growth Differ. 2020. PMID: 32112394 Free PMC article. Review.
Cited by
-
Decoding the olfactory map through targeted transcriptomics links murine olfactory receptors to glomeruli.Nat Commun. 2022 Sep 1;13(1):5137. doi: 10.1038/s41467-022-32267-3. Nat Commun. 2022. PMID: 36050313 Free PMC article.
-
Overlapping but distinct topology for zebrafish V2R-like olfactory receptors reminiscent of odorant receptor spatial expression zones.BMC Genomics. 2018 May 23;19(1):383. doi: 10.1186/s12864-018-4740-8. BMC Genomics. 2018. PMID: 29792162 Free PMC article.
-
Quadruple Immunostaining of the Olfactory Bulb for Visualization of Olfactory Sensory Axon Molecular Identity Codes.J Vis Exp. 2017 Jun 5;(124):55893. doi: 10.3791/55893. J Vis Exp. 2017. PMID: 28605383 Free PMC article.
-
Shaping the olfactory map: cell type-specific activity patterns guide circuit formation.Front Neural Circuits. 2024 May 27;18:1409680. doi: 10.3389/fncir.2024.1409680. eCollection 2024. Front Neural Circuits. 2024. PMID: 38860141 Free PMC article. Review.
-
Differential timing of neurogenesis underlies dorsal-ventral topographic projection of olfactory sensory neurons.Neural Dev. 2017 Feb 13;12(1):2. doi: 10.1186/s13064-017-0079-0. Neural Dev. 2017. PMID: 28193234 Free PMC article.
References
-
- Miyamichi K, Serizawa S, Kimura HM, Sakano H. Continuous and overlapping expression domains of odorant receptor genes in the olfactory epithelium determine the dorsal/ventral positioning of glomeruli in the olfactory bulb. J Neurosci. 2005;25:3586–3592. doi: 10.1523/JNEUROSCI.0324-05.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

