Specifying peripheral heterochromatin during nuclear lamina reassembly
- PMID: 24637393
- PMCID: PMC4028353
- DOI: 10.4161/nucl.28167
Specifying peripheral heterochromatin during nuclear lamina reassembly
Abstract
A conserved organizational feature of eukaryotic nuclei is the peripheral heterochromatin compartment, which provides a protected area for epigenetically silent genes and gene-poor DNA. In metazoan cells this compartment is associated with the nuclear lamina, the protein meshwork at the inner edge of the nucleus. Heterochromatin-nuclear lamina interactions promote epigenetic gene silencing, which may drive many normal and diseased biological processes. We recently obtained evidence that a previously unstudied human protein, PRR14, participates in the tethering of heterochromatin to the inner nuclear periphery. PRR14 associates with the nuclear lamina and attaches to heterochromatin through its binding partner, heterochromatin protein 1 (HP1). After disassembly early in mitosis, PRR14 reassembles in two steps, first binding to anaphase chromosomes through HP1, followed by association with the nuclear lamina in telophase. PRR14 may thereby play a role in specifying HP1-bound heterochromatin for reattachment to the nuclear lamina at mitotic exit. Here we review the relevant literature, summarize our initial work, and provide additional comments and findings.
Keywords: Proline-Rich 14; heterochromatin; heterochromatin protein 1; nuclear envelope; nuclear lamina.
Figures
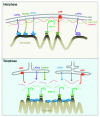
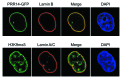
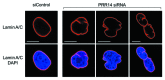
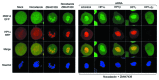

Comment on
- Poleshko A, Mansfield KM, Burlingame CC, Andrake MD, Shah NR, Katz RA. The human protein PRR14 tethers heterochromatin to the nuclear lamina during interphase and mitotic exit. Cell Rep. 2013;5:292–301. doi: 10.1016/j.celrep.2013.09.024.
Similar articles
-
The human protein PRR14 tethers heterochromatin to the nuclear lamina during interphase and mitotic exit.Cell Rep. 2013 Oct 31;5(2):292-301. doi: 10.1016/j.celrep.2013.09.024. Cell Rep. 2013. PMID: 24209742 Free PMC article.
-
PRR14 organizes H3K9me3-modified heterochromatin at the nuclear lamina.Nucleus. 2023 Dec;14(1):2165602. doi: 10.1080/19491034.2023.2165602. Nucleus. 2023. PMID: 36633363 Free PMC article.
-
The PRR14 heterochromatin tether encodes modular domains that mediate and regulate nuclear lamina targeting.J Cell Sci. 2020 May 27;133(10):jcs240416. doi: 10.1242/jcs.240416. J Cell Sci. 2020. PMID: 32317397 Free PMC article.
-
The secret life of chromatin tethers.FEBS Lett. 2023 Nov;597(22):2782-2790. doi: 10.1002/1873-3468.14685. Epub 2023 Jun 25. FEBS Lett. 2023. PMID: 37339933 Free PMC article. Review.
-
The Nuclear Lamina as an Organizer of Chromosome Architecture.Cells. 2019 Feb 8;8(2):136. doi: 10.3390/cells8020136. Cells. 2019. PMID: 30744037 Free PMC article. Review.
Cited by
-
PRR14 acts a novel oncogene activating the PI3K signal pathway in human cutaneous squamous cell carcinoma.J Cancer. 2023 May 21;14(9):1531-1540. doi: 10.7150/jca.83695. eCollection 2023. J Cancer. 2023. PMID: 37325059 Free PMC article.
-
Multiplex coherent anti-Stokes Raman scattering highlights state of chromatin condensation in CH region.Sci Rep. 2019 Sep 25;9(1):13862. doi: 10.1038/s41598-019-50453-0. Sci Rep. 2019. PMID: 31554897 Free PMC article.
-
Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation.Cell. 2014 Nov 6;159(4):884-95. doi: 10.1016/j.cell.2014.09.055. Epub 2014 Oct 30. Cell. 2014. PMID: 25417163 Free PMC article.
-
Mapping the micro-proteome of the nuclear lamina and lamina-associated domains.Life Sci Alliance. 2021 Mar 23;4(5):e202000774. doi: 10.26508/lsa.202000774. Print 2021 May. Life Sci Alliance. 2021. PMID: 33758005 Free PMC article.
-
The shifting shape of genomes: dynamics of heterochromatin interactions at the nuclear lamina.Curr Opin Genet Dev. 2021 Apr;67:163-173. doi: 10.1016/j.gde.2021.02.003. Epub 2021 Mar 25. Curr Opin Genet Dev. 2021. PMID: 33774266 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
