7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis
- PMID: 24637017
- PMCID: PMC5906793
- DOI: 10.1016/j.neulet.2014.02.058
7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis
Abstract
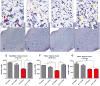
Amyotrophic lateral sclerosis (ALS) is an enigmatic neurodegenerative disorder without any effective treatment characterized by loss of motor neurons (MNs) that results in rapidly progressive motor weakness and early death due to respiratory failure. Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family known to play a prominent role in the differentiation and survival of MNs. The flavonoid 7,8-dihydroxyflavone (7,8-DHF) is a potent and selective small molecule tyrosine kinase receptor B (TrkB) agonist that mimics the effects of BDNF. In the present study, we evaluated the neuroprotective effects of 7,8-DHF in a transgenic ALS mouse model (SOD1(G93A)). We found that chronic administration of 7,8-DHF significantly improved motor deficits, and preserved spinal MNs count and dendritic spines in SOD1(G93A) mice. These data suggest that 7,8-DHF should be considered as a potential therapy for ALS and the other motor neuron diseases.
Keywords: 7,8-Dihydroxyflavone; Amyotrophic lateral sclerosis; BDNF; Motor neurons; TrkB.
Published by Elsevier Ireland Ltd.
Conflict of interest statement
Figures
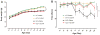

Similar articles
-
7,8-Dihydroxyflavone leads to survival of cultured embryonic motoneurons by activating intracellular signaling pathways.Mol Cell Neurosci. 2013 Sep;56:18-28. doi: 10.1016/j.mcn.2013.02.007. Epub 2013 Mar 14. Mol Cell Neurosci. 2013. PMID: 23500004
-
Sigma-1R agonist improves motor function and motoneuron survival in ALS mice.Neurotherapeutics. 2012 Oct;9(4):814-26. doi: 10.1007/s13311-012-0140-y. Neurotherapeutics. 2012. PMID: 22935988 Free PMC article.
-
Death receptor 6 (DR6) antagonist antibody is neuroprotective in the mouse SOD1G93A model of amyotrophic lateral sclerosis.Cell Death Dis. 2013 Oct 10;4(10):e841. doi: 10.1038/cddis.2013.378. Cell Death Dis. 2013. PMID: 24113175 Free PMC article.
-
Transgenic mice with human mutant genes causing Parkinson's disease and amyotrophic lateral sclerosis provide common insight into mechanisms of motor neuron selective vulnerability to degeneration.Rev Neurosci. 2007;18(2):115-36. doi: 10.1515/revneuro.2007.18.2.115. Rev Neurosci. 2007. PMID: 17593875 Review.
-
Brain-derived neurotrophic factor/tropomyosin receptor kinase B signaling in spinal muscular atrophy and amyotrophic lateral sclerosis.Neurobiol Dis. 2024 Jan;190:106377. doi: 10.1016/j.nbd.2023.106377. Epub 2023 Dec 12. Neurobiol Dis. 2024. PMID: 38092270 Review.
Cited by
-
Effectiveness of Flavonoid-Rich Diet in Alleviating Symptoms of Neurodegenerative Diseases.Foods. 2024 Jun 19;13(12):1931. doi: 10.3390/foods13121931. Foods. 2024. PMID: 38928874 Free PMC article. Review.
-
7, 8, 3'-Trihydroxyflavone Promotes Neurite Outgrowth and Protects Against Bupivacaine-Induced Neurotoxicity in Mouse Dorsal Root Ganglion Neurons.Med Sci Monit. 2016 Jul 2;22:2301-8. doi: 10.12659/msm.896961. Med Sci Monit. 2016. PMID: 27371503 Free PMC article.
-
A Small Molecule TrkB Neurotrophin Receptor Partial Agonist as Possible Treatment for Experimental Nonarteritic Anterior Ischemic Optic Neuropathy.Curr Eye Res. 2018 Dec;43(12):1489-1499. doi: 10.1080/02713683.2018.1508726. Epub 2018 Oct 1. Curr Eye Res. 2018. PMID: 30273053 Free PMC article.
-
Uncovering the Pharmacological Mechanism of Stemazole in the Treatment of Neurodegenerative Diseases Based on a Network Pharmacology Approach.Int J Mol Sci. 2020 Jan 9;21(2):427. doi: 10.3390/ijms21020427. Int J Mol Sci. 2020. PMID: 31936558 Free PMC article.
-
Regulated cell death: discovery, features and implications for neurodegenerative diseases.Cell Commun Signal. 2021 Dec 18;19(1):120. doi: 10.1186/s12964-021-00799-8. Cell Commun Signal. 2021. PMID: 34922574 Free PMC article. Review.
References
-
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. - PubMed
-
- Sathasivam S, Ince PG, Shaw PJ. Apoptosis in amyotrophic lateral sclerosis: a review of the evidence. Neuropathol Appl Neurobiol. 2001;27:257–274. - PubMed
-
- Cozzolino M, Ferri A, Carrì MT. Amyotrophic lateral sclerosis: from current developments in the laboratory to clinical implications. Antioxid Redox Signal. 2008;10:405–43. - PubMed
-
- Beleza-Meireles A, Al-Chalabi A. Genetic studies of amyotrophic lateral sclerosis: controversies and perspectives. Amyotroph Lateral Scler. 2009;10:1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

