Pyrrolysyl-tRNA synthetase: an ordinary enzyme but an outstanding genetic code expansion tool
- PMID: 24631543
- PMCID: PMC4016821
- DOI: 10.1016/j.bbapap.2014.03.002
Pyrrolysyl-tRNA synthetase: an ordinary enzyme but an outstanding genetic code expansion tool
Abstract
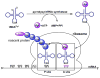
The genetic incorporation of the 22nd proteinogenic amino acid, pyrrolysine (Pyl) at amber codon is achieved by the action of pyrrolysyl-tRNA synthetase (PylRS) together with its cognate tRNA(Pyl). Unlike most aminoacyl-tRNA synthetases, PylRS displays high substrate side chain promiscuity, low selectivity toward its substrate α-amine, and low selectivity toward the anticodon of tRNA(Pyl). These unique but ordinary features of PylRS as an aminoacyl-tRNA synthetase allow the Pyl incorporation machinery to be easily engineered for the genetic incorporation of more than 100 non-canonical amino acids (NCAAs) or α-hydroxy acids into proteins at amber codon and the reassignment of other codons such as ochre UAA, opal UGA, and four-base AGGA codons to code NCAAs.
Keywords: Amber codon; Genetic code expansion; Hydroxy acids; Non-canonical amino acids; Pyrrolysine.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
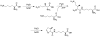


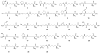


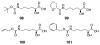
Similar articles
-
tRNAPyl: Structure, function, and applications.RNA Biol. 2018;15(4-5):441-452. doi: 10.1080/15476286.2017.1356561. Epub 2017 Sep 13. RNA Biol. 2018. PMID: 28837402 Free PMC article. Review.
-
Update of the Pyrrolysyl-tRNA Synthetase/tRNAPyl Pair and Derivatives for Genetic Code Expansion.J Bacteriol. 2023 Feb 22;205(2):e0038522. doi: 10.1128/jb.00385-22. Epub 2023 Jan 25. J Bacteriol. 2023. PMID: 36695595 Free PMC article. Review.
-
Mutually orthogonal pyrrolysyl-tRNA synthetase/tRNA pairs.Nat Chem. 2018 Aug;10(8):831-837. doi: 10.1038/s41557-018-0052-5. Epub 2018 May 28. Nat Chem. 2018. PMID: 29807989 Free PMC article.
-
Recognition of non-alpha-amino substrates by pyrrolysyl-tRNA synthetase.J Mol Biol. 2009 Feb 6;385(5):1352-60. doi: 10.1016/j.jmb.2008.11.059. Epub 2008 Dec 11. J Mol Biol. 2009. PMID: 19100747
-
Structural Basis for Genetic-Code Expansion with Bulky Lysine Derivatives by an Engineered Pyrrolysyl-tRNA Synthetase.Cell Chem Biol. 2019 Jul 18;26(7):936-949.e13. doi: 10.1016/j.chembiol.2019.03.008. Epub 2019 Apr 25. Cell Chem Biol. 2019. PMID: 31031143
Cited by
-
Noncanonical Amino Acids in Biocatalysis.Chem Rev. 2024 Jul 24;124(14):8740-8786. doi: 10.1021/acs.chemrev.4c00120. Epub 2024 Jul 3. Chem Rev. 2024. PMID: 38959423 Free PMC article. Review.
-
Expanding the chemical diversity of M13 bacteriophage.Front Microbiol. 2022 Aug 8;13:961093. doi: 10.3389/fmicb.2022.961093. eCollection 2022. Front Microbiol. 2022. PMID: 36003937 Free PMC article. Review.
-
The physical basis and practical consequences of biological promiscuity.Phys Biol. 2020 Apr 3:10.1088/1478-3975/ab8697. doi: 10.1088/1478-3975/ab8697. Online ahead of print. Phys Biol. 2020. PMID: 32244231 Free PMC article.
-
Reducing the genetic code induces massive rearrangement of the proteome.Proc Natl Acad Sci U S A. 2014 Dec 2;111(48):17206-11. doi: 10.1073/pnas.1420193111. Epub 2014 Nov 17. Proc Natl Acad Sci U S A. 2014. PMID: 25404328 Free PMC article.
-
Noncanonical Amino Acid Incorporation in Mice.Methods Mol Biol. 2023;2676:265-284. doi: 10.1007/978-1-0716-3251-2_19. Methods Mol Biol. 2023. PMID: 37277639
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

