Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation
- PMID: 24630723
- PMCID: PMC4034535
- DOI: 10.1016/j.cell.2014.01.063
Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation
Abstract
Pathogens and cellular danger signals activate sensors such as RIG-I and NLRP3 to produce robust immune and inflammatory responses through respective adaptor proteins MAVS and ASC, which harbor essential N-terminal CARD and PYRIN domains, respectively. Here, we show that CARD and PYRIN function as bona fide prions in yeast and that their prion forms are inducible by their respective upstream activators. Likewise, a yeast prion domain can functionally replace CARD and PYRIN in mammalian cell signaling. Mutations in MAVS and ASC that disrupt their prion activities in yeast also abrogate their ability to signal in mammalian cells. Furthermore, fibers of recombinant PYRIN can convert ASC into functional polymers capable of activating caspase-1. Remarkably, a conserved fungal NOD-like receptor and prion pair can functionally reconstitute signaling of NLRP3 and ASC PYRINs in mammalian cells. These results indicate that prion-like polymerization is a conserved signal transduction mechanism in innate immunity and inflammation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

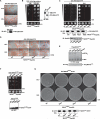

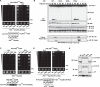


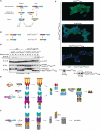
Comment in
-
Inflammasome: putting the pieces together.Cell. 2014 Mar 13;156(6):1127-1129. doi: 10.1016/j.cell.2014.02.038. Cell. 2014. PMID: 24630715
-
Inflammasomes: polymeric assembly.Nat Rev Immunol. 2014 May;14(5):287. doi: 10.1038/nri3669. Epub 2014 Apr 11. Nat Rev Immunol. 2014. PMID: 24722480 No abstract available.
Similar articles
-
Inflammasome: putting the pieces together.Cell. 2014 Mar 13;156(6):1127-1129. doi: 10.1016/j.cell.2014.02.038. Cell. 2014. PMID: 24630715
-
The CARD plays a critical role in ASC foci formation and inflammasome signalling.Biochem J. 2013 Feb 1;449(3):613-21. doi: 10.1042/BJ20121198. Biochem J. 2013. PMID: 23110696 Free PMC article.
-
Prion-Like Polymerization in Immunity and Inflammation.Cold Spring Harb Perspect Biol. 2017 Apr 3;9(4):a023580. doi: 10.1101/cshperspect.a023580. Cold Spring Harb Perspect Biol. 2017. PMID: 27881448 Free PMC article. Review.
-
The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation.Cell. 2013 Apr 11;153(2):348-61. doi: 10.1016/j.cell.2013.02.054. Cell. 2013. PMID: 23582325 Free PMC article.
-
Mechanisms of NOD-like receptor-associated inflammasome activation.Immunity. 2013 Sep 19;39(3):432-41. doi: 10.1016/j.immuni.2013.08.037. Immunity. 2013. PMID: 24054327 Free PMC article. Review.
Cited by
-
Prions, amyloids, and RNA: Pieces of a puzzle.Prion. 2016 May 3;10(3):182-206. doi: 10.1080/19336896.2016.1181253. Prion. 2016. PMID: 27248002 Free PMC article. Review.
-
Nucleic acid-induced inflammation on hematopoietic stem cells.Exp Hematol. 2024 Mar;131:104148. doi: 10.1016/j.exphem.2023.104148. Epub 2023 Dec 25. Exp Hematol. 2024. PMID: 38151171
-
Inflammasomes and its importance in viral infections.Immunol Res. 2016 Dec;64(5-6):1101-1117. doi: 10.1007/s12026-016-8873-z. Immunol Res. 2016. PMID: 27699580 Review.
-
Innate Immune Sensing of Influenza A Virus.Viruses. 2020 Jul 14;12(7):755. doi: 10.3390/v12070755. Viruses. 2020. PMID: 32674269 Free PMC article. Review.
-
ATP-Binding and Hydrolysis in Inflammasome Activation.Molecules. 2020 Oct 7;25(19):4572. doi: 10.3390/molecules25194572. Molecules. 2020. PMID: 33036374 Free PMC article. Review.
References
-
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

