A cortical circuit for gain control by behavioral state
- PMID: 24630718
- PMCID: PMC4041382
- DOI: 10.1016/j.cell.2014.01.050
A cortical circuit for gain control by behavioral state
Abstract
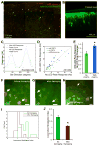
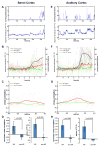
The brain's response to sensory input is strikingly modulated by behavioral state. Notably, the visual response of mouse primary visual cortex (V1) is enhanced by locomotion, a tractable and accessible example of a time-locked change in cortical state. The neural circuits that transmit behavioral state to sensory cortex to produce this modulation are unknown. In vivo calcium imaging of behaving animals revealed that locomotion activates vasoactive intestinal peptide (VIP)-positive neurons in mouse V1 independent of visual stimulation and largely through nicotinic inputs from basal forebrain. Optogenetic activation of VIP neurons increased V1 visual responses in stationary awake mice, artificially mimicking the effect of locomotion, and photolytic damage of VIP neurons abolished the enhancement of V1 responses by locomotion. These findings establish a cortical circuit for the enhancement of visual response by locomotion and provide a potential common circuit for the modulation of sensory processing by behavioral state.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
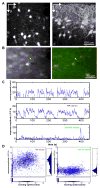
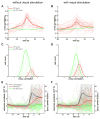

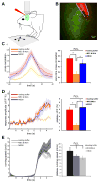
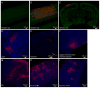
Comment in
-
Visual circuits get the VIP treatment.Cell. 2014 Mar 13;156(6):1123-1124. doi: 10.1016/j.cell.2014.02.043. Cell. 2014. PMID: 24630713
Similar articles
-
Behavioral-state modulation of inhibition is context-dependent and cell type specific in mouse visual cortex.Elife. 2016 Aug 23;5:e14985. doi: 10.7554/eLife.14985. Elife. 2016. PMID: 27552056 Free PMC article.
-
Visual processing mode switching regulated by VIP cells.Sci Rep. 2017 May 12;7(1):1843. doi: 10.1038/s41598-017-01830-0. Sci Rep. 2017. PMID: 28500299 Free PMC article.
-
Effects of Locomotion in Auditory Cortex Are Not Mediated by the VIP Network.Front Neural Circuits. 2021 Apr 7;15:618881. doi: 10.3389/fncir.2021.618881. eCollection 2021. Front Neural Circuits. 2021. PMID: 33897378 Free PMC article.
-
Stimulus-specific enhancement in mouse visual cortex requires GABA but not VIP-peptide release from VIP interneurons.J Neurophysiol. 2024 Jul 1;132(1):34-44. doi: 10.1152/jn.00463.2023. Epub 2024 May 22. J Neurophysiol. 2024. PMID: 38774975 Free PMC article.
-
A Neural Circuit That Controls Cortical State, Plasticity, and the Gain of Sensory Responses in Mouse.Cold Spring Harb Symp Quant Biol. 2014;79:1-9. doi: 10.1101/sqb.2014.79.024927. Epub 2015 May 6. Cold Spring Harb Symp Quant Biol. 2014. PMID: 25948638 Free PMC article. Review.
Cited by
-
Organization of long-range inputs and outputs of frontal cortex for top-down control.Nat Neurosci. 2016 Dec;19(12):1733-1742. doi: 10.1038/nn.4417. Epub 2016 Oct 17. Nat Neurosci. 2016. PMID: 27749828 Free PMC article.
-
Sensory inputs control the integration of neurogliaform interneurons into cortical circuits.Nat Neurosci. 2015 Mar;18(3):393-401. doi: 10.1038/nn.3946. Epub 2015 Feb 9. Nat Neurosci. 2015. PMID: 25664912 Free PMC article.
-
Neuronal networks and mediators of cortical neurovascular coupling responses in normal and altered brain states.Philos Trans R Soc Lond B Biol Sci. 2016 Oct 5;371(1705):20150350. doi: 10.1098/rstb.2015.0350. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27574304 Free PMC article. Review.
-
Cortex-wide response mode of VIP-expressing inhibitory neurons by reward and punishment.Elife. 2022 Nov 23;11:e78815. doi: 10.7554/eLife.78815. Elife. 2022. PMID: 36416886 Free PMC article.
-
Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto CA1 pyramidal neurons shapes hippocampal output.Nat Commun. 2020 Sep 2;11(1):4395. doi: 10.1038/s41467-020-18074-8. Nat Commun. 2020. PMID: 32879322 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

