Comparison of survival outcomes among cancer patients treated in and out of clinical trials
- PMID: 24627276
- PMCID: PMC3982777
- DOI: 10.1093/jnci/dju002
Comparison of survival outcomes among cancer patients treated in and out of clinical trials
Abstract
Background: Clinical trials test the efficacy of a treatment in a select patient population. We examined whether cancer clinical trial patients were similar to nontrial, "real-world" patients with respect to presenting characteristics and survival.
Methods: We reviewed the SWOG national clinical trials consortium database to identify candidate trials. Demographic factors, stage, and overall survival for patients in the standard arms were compared with nontrial control subjects selected from the Surveillance, Epidemiology, and End Results program. Multivariable survival analyses using Cox regression were conducted. The survival functions from aggregate data across all studies were compared separately by prognosis (≥50% vs <50% average 2-year survival). All statistical tests were two-sided.
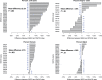
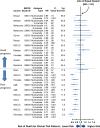
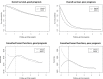
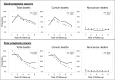
Results: We analyzed 21 SWOG studies (11 good prognosis and 10 poor prognosis) comprising 5190 patients enrolled from 1987 to 2007. Trial patients were younger than nontrial patients (P < .001). In multivariable analysis, trial participation was not associated with improved overall survival for all 11 good-prognosis studies but was associated with better survival for nine of 10 poor-prognosis studies (P < .001). The impact of trial participation on overall survival endured for only 1 year.
Conclusions: Trial participation was associated with better survival in the first year after diagnosis, likely because of eligibility criteria that excluded higher comorbidity patients from trials. Similar survival patterns between trial and nontrial patients after the first year suggest that trial standard arm outcomes are generalizable over the long term and may improve confidence that trial treatment effects will translate to the real-world setting. Reducing eligibility criteria would improve access to clinical trials.
Figures

Comment in
-
In and out, good and bad news, of generalizability of SWOG treatment trial results.J Natl Cancer Inst. 2014 Mar;106(3):dju027. doi: 10.1093/jnci/dju027. Epub 2014 Mar 13. J Natl Cancer Inst. 2014. PMID: 24627277 No abstract available.
Similar articles
-
Do Patients With Multiple Myeloma Enrolled in Clinical Trials Live Longer?Am J Clin Oncol. 2021 Dec 1;44(12):603-612. doi: 10.1097/COC.0000000000000873. Am J Clin Oncol. 2021. PMID: 34753885
-
Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference?J Clin Oncol. 2011 Oct 20;29(30):4045-53. doi: 10.1200/JCO.2011.36.2954. Epub 2011 Sep 19. J Clin Oncol. 2011. PMID: 21931022 Free PMC article.
-
Geographic Distribution and Survival Outcomes for Rural Patients With Cancer Treated in Clinical Trials.JAMA Netw Open. 2018 Aug 3;1(4):e181235. doi: 10.1001/jamanetworkopen.2018.1235. JAMA Netw Open. 2018. PMID: 30646114 Free PMC article.
-
Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. PMID: 30354042 Free Books & Documents. Review.
-
Screening for Cervical Cancer With High-Risk Human Papillomavirus Testing: A Systematic Evidence Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Aug. Report No.: 17-05231-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Aug. Report No.: 17-05231-EF-1. PMID: 30256575 Free Books & Documents. Review.
Cited by
-
Use and Effectiveness of Neoadjuvant Chemotherapy for Treatment of Ovarian Cancer.J Clin Oncol. 2016 Nov 10;34(32):3854-3863. doi: 10.1200/JCO.2016.68.1239. J Clin Oncol. 2016. PMID: 27601552 Free PMC article.
-
Envisioning clinical trials as complex interventions.Cancer. 2022 Sep 1;128(17):3145-3151. doi: 10.1002/cncr.34357. Epub 2022 Jun 29. Cancer. 2022. PMID: 35766902 Free PMC article.
-
Professional educational needs for chemotherapy-induced nausea and vomiting (CINV): multinational survey results from 2388 health care providers.Support Care Cancer. 2015 Jan;23(1):151-7. doi: 10.1007/s00520-014-2325-x. Support Care Cancer. 2015. PMID: 25015057 Free PMC article.
-
Comparison of Clinical Characteristics Between Clinical Trial Participants and Nonparticipants Using Electronic Health Record Data.JAMA Netw Open. 2021 Apr 1;4(4):e214732. doi: 10.1001/jamanetworkopen.2021.4732. JAMA Netw Open. 2021. PMID: 33825838 Free PMC article.
-
Adult leukemia survival trends in the United States by subtype: A population-based registry study of 370,994 patients diagnosed during 1995-2009.Cancer. 2018 Oct 1;124(19):3856-3867. doi: 10.1002/cncr.31674. Epub 2018 Oct 21. Cancer. 2018. PMID: 30343495 Free PMC article.
References
-
- Tejada HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88(12):812–816 - PubMed
-
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726 - PubMed
-
- Ford JG, Howerton HW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–242 - PubMed
-
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–1156 - PubMed
-
- Unger JM, Green S, Albain KS. Under-representation of elderly patients in cancer clinical trials: causes and remedial strategies. In: Balducci L, Lyman GH, Ershler WB, Extermann M, eds. Comprehensive Geriatric Oncology. 2nd ed. Taylor and Francis; 2004:464–491
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

