Preferential and comprehensive reconstitution of severely damaged sciatic nerve using murine skeletal muscle-derived multipotent stem cells
- PMID: 24614849
- PMCID: PMC3948784
- DOI: 10.1371/journal.pone.0091257
Preferential and comprehensive reconstitution of severely damaged sciatic nerve using murine skeletal muscle-derived multipotent stem cells
Abstract
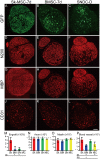
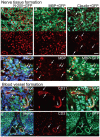
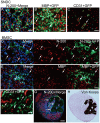
Loss of vital functions in the somatic motor and sensory nervous systems can be induced by severe peripheral nerve transection with a long gap following trauma. In such cases, autologous nerve grafts have been used as the gold standard, with the expectation of activation and proliferation of graft-concomitant Schwann cells associated with their paracrine effects. However, there are a limited number of suitable sites available for harvesting of nerve autografts due to the unavoidable sacrifice of other healthy functions. To overcome this problem, the potential of skeletal muscle-derived multipotent stem cells (Sk-MSCs) was examined as a novel alternative cell source for peripheral nerve regeneration. Cultured/expanded Sk-MSCs were injected into severely crushed sciatic nerve corresponding to serious neurotmesis. After 4 weeks, engrafted Sk-MSCs preferentially differentiated into not only Schwann cells, but also perineurial/endoneurial cells, and formed myelin sheath and perineurium/endoneurium, encircling the regenerated axons. Increased vascular formation was also observed, leading to a favorable blood supply and waste product excretion. In addition, engrafted cells expressed key neurotrophic and nerve/vascular growth factor mRNAs; thus, endocrine/paracrine effects for the donor/recipient cells were also expected. Interestingly, skeletal myogenic capacity of expanded Sk-MSCs was clearly diminished in peripheral nerve niche. The same differentiation and tissue reconstitution capacity of Sk-MSCs was sufficiently exerted in the long nerve gap bridging the acellular conduit, which facilitated nerve regeneration/reconnection. These effects represent favorable functional recovery in Sk-MSC-treated mice, as demonstrated by good corduroy walking. We also demonstrated that these differentiation characteristics of the Sk-MSCs were comparable to native peripheral nerve-derived cells, whereas the therapeutic capacities were largely superior in Sk-MSCs. Therefore, Sk-MSCs can be a novel/suitable alternative cell source for healthy nerve autografts.
Conflict of interest statement
Figures




Similar articles
-
A Long-Gap Peripheral Nerve Injury Therapy Using Human Skeletal Muscle-Derived Stem Cells (Sk-SCs): An Achievement of Significant Morphological, Numerical and Functional Recovery.PLoS One. 2016 Nov 15;11(11):e0166639. doi: 10.1371/journal.pone.0166639. eCollection 2016. PLoS One. 2016. PMID: 27846318 Free PMC article.
-
3D reconstitution of nerve-blood vessel networks using skeletal muscle-derived multipotent stem cell sheet pellets.Regen Med. 2013 Jul;8(4):437-51. doi: 10.2217/rme.13.30. Regen Med. 2013. PMID: 23826698
-
Bridging long gap peripheral nerve injury using skeletal muscle-derived multipotent stem cells.Neural Regen Res. 2014 Jul 15;9(14):1333-6. doi: 10.4103/1673-5374.137582. Neural Regen Res. 2014. PMID: 25221587 Free PMC article. Review.
-
Reconstruction of Multiple Facial Nerve Branches Using Skeletal Muscle-Derived Multipotent Stem Cell Sheet-Pellet Transplantation.PLoS One. 2015 Sep 15;10(9):e0138371. doi: 10.1371/journal.pone.0138371. eCollection 2015. PLoS One. 2015. PMID: 26372044 Free PMC article.
-
Applications of Proteomics to Nerve Regeneration Research.In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. PMID: 21882439 Free Books & Documents. Review.
Cited by
-
Reconstitution of the complete rupture in musculotendinous junction using skeletal muscle-derived multipotent stem cell sheet-pellets as a "bio-bond".PeerJ. 2016 Jul 19;4:e2231. doi: 10.7717/peerj.2231. eCollection 2016. PeerJ. 2016. PMID: 27547541 Free PMC article.
-
Skeletal muscle transcriptomics identifies common pathways in nerve crush injury and ageing.Skelet Muscle. 2022 Jan 29;12(1):3. doi: 10.1186/s13395-021-00283-4. Skelet Muscle. 2022. PMID: 35093178 Free PMC article.
-
Ultrasound imaging of chitosan nerve conduits that bridge sciatic nerve defects in rats.Neural Regen Res. 2014 Jul 15;9(14):1386-8. doi: 10.4103/1673-5374.137592. Neural Regen Res. 2014. PMID: 25221596 Free PMC article. No abstract available.
-
The Current Role of Stem Cells in Orthopaedic Surgery.Malays Orthop J. 2015 Nov;9(3):1-7. doi: 10.5704/MOJ.1511.016. Malays Orthop J. 2015. PMID: 28611902 Free PMC article. Review.
-
Differentiation Capacity of Porcine Skeletal Muscle-Derived Stem Cells as Intermediate Species between Mice and Humans.Int J Mol Sci. 2023 Jun 7;24(12):9862. doi: 10.3390/ijms24129862. Int J Mol Sci. 2023. PMID: 37373009 Free PMC article.
References
-
- Robinson LR (2000) Traumatic injury to peripheral nerves. Muscle Nerve 23: 863–873. - PubMed
-
- Robinson PP, Boissonade FM, Loescher AR, Smith KG, Yates JM, et al. (2004) Peripheral mechanisms for the initiation of pain following trigeminal nerve injury. J Orofac Pain 18: 287–292. - PubMed
-
- Sunderland S (1990) The anatomy and physiology of nerve injury. Muscle Nerve 13: 771–784. - PubMed
-
- Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, et al. (2011) Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng 39: 81–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

