Attenuation of hedgehog acyltransferase-catalyzed sonic Hedgehog palmitoylation causes reduced signaling, proliferation and invasiveness of human carcinoma cells
- PMID: 24608521
- PMCID: PMC3946499
- DOI: 10.1371/journal.pone.0089899
Attenuation of hedgehog acyltransferase-catalyzed sonic Hedgehog palmitoylation causes reduced signaling, proliferation and invasiveness of human carcinoma cells
Abstract
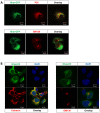
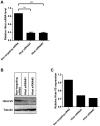
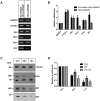
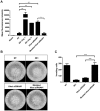
Overexpression of Hedgehog family proteins contributes to the aetiology of many cancers. To be highly active, Hedgehog proteins must be palmitoylated at their N-terminus by the MBOAT family multispanning membrane enzyme Hedgehog acyltransferase (Hhat). In a pancreatic ductal adenocarcinoma (PDAC) cell line PANC-1 and transfected HEK293a cells Hhat localized to the endoplasmic reticulum. siRNA knockdown showed that Hhat is required for Sonic hedgehog (Shh) palmitoylation, for its assembly into high molecular weight extracellular complexes and for functional activity. Hhat knockdown inhibited Hh autocrine and juxtacrine signaling, and inhibited PDAC cell growth and invasiveness in vitro. In addition, Hhat knockdown in a HEK293a cell line constitutively expressing Shh and A549 human non-small cell lung cancer cells inhibited their ability to signal in a juxtacrine/paracrine fashion to the reporter cell lines C3H10T1/2 and Shh-Light2. Our data identify Hhat as a key player in Hh-dependent signaling and tumour cell transformed behaviour.
Conflict of interest statement
Figures

Similar articles
-
Membrane topology of hedgehog acyltransferase.J Biol Chem. 2015 Jan 23;290(4):2235-43. doi: 10.1074/jbc.M114.625764. Epub 2014 Dec 8. J Biol Chem. 2015. PMID: 25488661 Free PMC article.
-
Palmitoylation of Hedgehog proteins by Hedgehog acyltransferase: roles in signalling and disease.Open Biol. 2021 Mar;11(3):200414. doi: 10.1098/rsob.200414. Epub 2021 Mar 3. Open Biol. 2021. PMID: 33653085 Free PMC article. Review.
-
Identification of conserved regions and residues within Hedgehog acyltransferase critical for palmitoylation of Sonic Hedgehog.PLoS One. 2010 Jun 23;5(6):e11195. doi: 10.1371/journal.pone.0011195. PLoS One. 2010. PMID: 20585641 Free PMC article.
-
Hedgehog Acyltransferase Promotes Uptake of Palmitoyl-CoA across the Endoplasmic Reticulum Membrane.Cell Rep. 2019 Dec 24;29(13):4608-4619.e4. doi: 10.1016/j.celrep.2019.11.110. Cell Rep. 2019. PMID: 31875564 Free PMC article.
-
Palmitoylation of Hedgehog proteins.Vitam Horm. 2012;88:229-52. doi: 10.1016/B978-0-12-394622-5.00010-9. Vitam Horm. 2012. PMID: 22391306 Free PMC article. Review.
Cited by
-
Membrane topology of hedgehog acyltransferase.J Biol Chem. 2015 Jan 23;290(4):2235-43. doi: 10.1074/jbc.M114.625764. Epub 2014 Dec 8. J Biol Chem. 2015. PMID: 25488661 Free PMC article.
-
A rising tide lifts all MBOATs: recent progress in structural and functional understanding of membrane bound O-acyltransferases.Front Physiol. 2023 May 4;14:1167873. doi: 10.3389/fphys.2023.1167873. eCollection 2023. Front Physiol. 2023. PMID: 37250116 Free PMC article. Review.
-
Gelatin methacryloyl and Laponite bioink for 3D bioprinted organotypic tumor modeling.Biofabrication. 2023 Jul 20;15(4):10.1088/1758-5090/ace0db. doi: 10.1088/1758-5090/ace0db. Biofabrication. 2023. PMID: 37348491 Free PMC article.
-
Design, Synthesis, and Evaluation of Inhibitors of Hedgehog Acyltransferase.J Med Chem. 2024 Jan 25;67(2):1061-1078. doi: 10.1021/acs.jmedchem.3c01363. Epub 2024 Jan 10. J Med Chem. 2024. PMID: 38198226 Free PMC article.
-
Hedgehog-Interacting Protein is a multimodal antagonist of Hedgehog signalling.Nat Commun. 2021 Dec 9;12(1):7171. doi: 10.1038/s41467-021-27475-2. Nat Commun. 2021. PMID: 34887403 Free PMC article.
References
-
- Varjosalo M, Taipale J (2008) Hedgehog: functions and mechanisms. Genes Dev 22: 2454–2472. - PubMed
-
- Adolphe C, Narang M, Ellis T, Wicking C, Kaur P, et al. (2004) An in vivo comparative study of sonic, desert and Indian hedgehog reveals that hedgehog pathway activity regulates epidermal stem cell homeostasis. Development 131: 5009–5019. - PubMed
-
- di Magliano PM, Hebrok M (2003) Hedgehog signaling in cancer formation and maintenance. Nat Rev Cancer 3: 903–911. - PubMed
-
- Katano M (2005) Hedgehog signaling pathway as a therapeutic target in breast cancer. Cancer Lett 227: 99–104. - PubMed
-
- Hatsell S, Frost AR (2007) Hedgehog signaling in mammary gland development and breast cancer. J Mamm Gland Biol Neoplasia 12: 163–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

